Dermacentor marginatus is an ixodid tick that has a heteroxenous life cycle and a variety of hosts including canines, horses, and humans [
97,
98]. It is a tick with a cosmopolitan distribution, present mainly in the Nearctic, Palearctic, and Neotropic ecozones of the planet [
99,
100,
101]. In the search for proteins for the development of a vaccine against
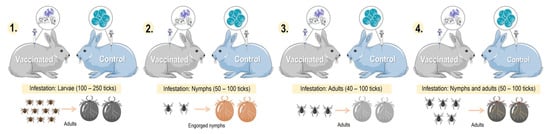
D. marginatus, the New Zealand white rabbit was used as an animal model in infestations and vaccination experiments. A study infested New Zealand breed rabbits with
D. marginatus after administering the last dose of the immunogen of GST, recording moderate vaccine efficacy against
D. marginatus (
Figure 5) [
30].
Figure 5. Comparison of different models of tick infestation in rabbits: 1. larval-stage tick infestation; 2. nymphal-stage tick infestation; 3. adult-stage tick infestation; and 4. nymphal- and adult-stage tick infestation. Parts of the figures were drawn by using pictures from Servier Medical Art:
http://smart.servier.com/ (accessed on 21 February 2023).
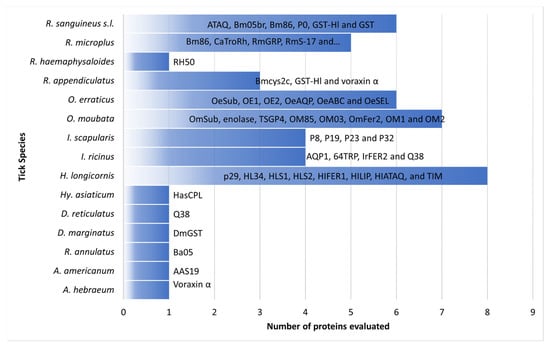
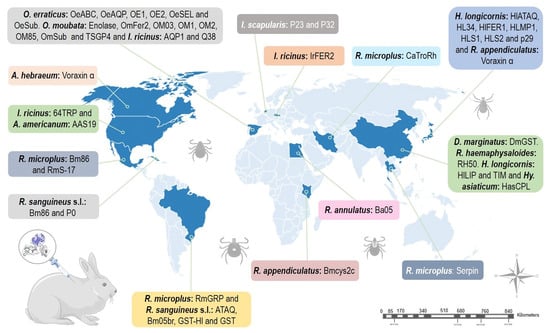
3. Summary
To date, 57 tick-derived proteins have been evaluated as potential anti-tick vaccines by studying the immunogenic responses generated using rabbits as an experimental model. Rabbit models for anti-tick vaccination trials have allowed for a better understanding of the physiological mechanisms of ticks infesting mammal hosts. For example, a study of the serpins HLS1, rHLS2, rSerpin, and RmS-17 in rabbits stimulated an immune response that affected the prolonged duration of feeding, increased mortality, and reduced oviposition in ticks like
H. longicornis and
R. microplus [
47,
64,
87,
102].
Globally, the use of rabbits has provided novel evidence on a vaccine based on salivary glycine-rich proteins in various medically important tick species. According to the findings obtained by Zhou et al., using rabbits immunized with the glycine-rich protein RH50, the protein was only expressed in the salivary glands of partially fed ticks and not in the salivary glands of unfed ticks or in the midgut, fat body, or ovary of partially fed ticks, in contrast to what was previously reported for p29 and Bm86 proteins [
63,
88,
121].
Rabbits have been used as an immunization model to evaluate immunological responses to a given antigen (Q38, Bm86, GST, serpins and voraxin) against different tick species. For example, high vaccine efficacy against both
I. ricinus and
D. reticulatus was obtained with the chimeric protein Q38 containing subolesin/akirin [
39].
Similarly, experiments on rabbits using voraxin α, a protein derived from the male tick and transferred to the female through copulation to stimulate female blood-feeding [
104], have yielded vaccine efficiency by reducing feeding times in
Amblyomma hebraeum. There is an amino acid sequence similarity between the voraxin α of
A. hebraeum (85%) and that of
D. variabilis (92%) and
R. appendiculatus (85%) [
86]. The immunization results could therefore potentially be similar, making this protein a good multispecies vaccine candidate.
The use of rabbits as animal models in the discovery of anti-tick molecules has been fundamental in enabling the testing of these molecules before inoculation of the natural hosts. It was verified that rabbits present an immune response similar to that of the natural hosts. For example, the use of the ferritin 2 protein to immunize rabbits infested with
I. ricinus (IrFER2) yielded an efficiency of 98%, while the efficiency of the same protein used in bovines infested with
R. microplus and
R. annulatus (RmFER2) was 64% and 72%, respectively [
96].
The immune responses generated by the different proteins studied in rabbits could vary depending on the challenges of ticks in immature or mature life stages. For example, the response generated by the p29 and HL34 proteins in the life stages of larvae, nymphs, and adults of
H. longicornis fed on immunized rabbits suggests that these proteins may be involved in mediating key physiological functions in the tick [
63,
71]. Although mature and immature ticks commonly express native p29, their sensitivities to rabbit immune responses against rp29 appear to be different [
63], while the native HL34 is expressed in both immature (larvae and nymphs) and adult ticks. It is thus likely that immunity against rHL34 is directed towards immature and mature ticks [
71].
Additionally, studies on rabbits have allowed us to broaden our knowledge about the “exposed” and “hidden” antigens of anti-tick proteins. For example, it was reported that HLS1 acts on the expression of hidden antigens, inhibiting the secretion of rHLS1 in rabbits during feeding [
64]. Also, 64TRP isoforms were characterized as “dual-acting” anti-tick proteins against
R. sanguineus s.l. and
I. ricinus; they target both “exposed” and “hidden” antigens, preventing attachment, and feeding by affecting the feeding site, as well as cross-reacting with ‘hidden’ midgut antigens, resulting in the death of engorged ticks [
48].
Results obtained from the study of the tick saliva proteome have shown a variety of proteins that protect ticks against host immune responses and antihemostatic mechanisms [
131,
132,
133,
134,
135,
136]. This is because, during hematophagy, tick salivary glands undergo remarkable growth and differentiation, accompanied by a significant increase in the synthesis of different proteins [
137]. Tirloni et al. identified 187 tick and 68 bovine proteins in the saliva proteome of
R. microplus, demonstrating that
R. microplus saliva is rich in hemolipoproteins, lipocalins, peptidase inhibitors, antimicrobial peptides, glycine, and maintenance proteins [
133]. These proteins, together with pharmacological bioactive lipids, can counteract the host’s defenses and hemostatic mechanisms [
131,
138], while the host physiological systems can trigger changes in the feeding activity of ticks [
139] by stimulating proteins to limit host defense mechanisms [
140].