
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hao Tong | -- | 2514 | 2023-09-06 12:01:50 | | | |
| 2 | Peter Tang | Meta information modification | 2514 | 2023-09-07 03:12:49 | | |
Video Upload Options
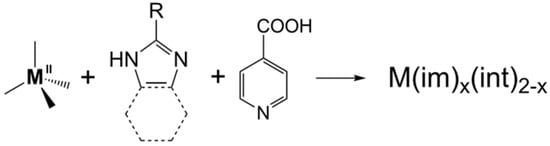
Zeolitic imidazolate frameworks (ZIFs) are an important subclass of metal–organic frameworks (MOFs). A new kind of MOF, namely tetrahedral imidazolate frameworks with auxiliary ligands (TIF-Ax) was reported, by adding linear ligands (Hint) into the zinc–imidazolate system. Introducing linear ligands into the M2+-imidazolate system overcomes the limitation of imidazole derivatives. Thanks to the synergistic effect of two different types of ligands, a series of new TIF-Ax with interesting topologies and a special pore environment has been reported, and they have attracted extensive attention in gas adsorption, separation, catalysis, heavy metal ion capture, and so on.
1. Introduction
|
Name |
Formula |
Space Group |
Topology |
Ref. |
|---|---|---|---|---|
|
TIF-A1 |
[Zn(ad)(int)](DMF) 1 |
Pna21 |
dmp |
[22] |
|
TIF-A2 |
Zn2(im)3(int) 2 |
Pca21 |
dia |
[22] |
|
TIF-A3 |
Zn2(im)(int)2(OH) |
C2/c |
neb |
[22] |
|
2-NH2-TIF-A1 |
[Zn(ad)(2-NH2-int)](DMF) |
Pna21 |
dmp |
[58] |
|
3-NH2-TIF-A1 |
[Zn(3-NH2-int)(ad)](DMF) [Zn(3-NH2-int)(ad)](DMA) 3 |
Pna21 |
dmp |
[59] |
|
Zn-thp-nit |
[Zn(thp)(nit)] 4 |
Pbca |
— |
[60] |
|
Cd-ad-int |
[Cd2(ad)2(int)2(DMF) (H2O)](DMF) |
P21/n |
mog |
[61] |
|
TIF-A4 |
Zn(im)(Ac) 5 |
Ima2 |
dia |
[62] |
|
TIF-A5 |
Zn(2-mim)(Ac) 6 |
P21/c |
sql |
[62] |
|
TIF-A6 |
Zn(2-eim)(Ac) 7 |
P21/c |
sql |
[62] |
|
TIF-A7 |
Zn(2-pim)(Ac) 8 |
Pna21 |
sql |
[62] |
|
TIF-A8 |
[Zn2(OH-)(Ac)(2-cim)2](DMSO) 9 |
Cmc21 |
sql |
[62] |
1 ad = adeninate; int = isonicotinate; DMF = N,N′-dimethylformamide; 2 im = imidazolate; 3 DMA = N,N′-dimethylacetamide; 4 thp = theophylline; nit = nicotinic acid; 5 Ac = acetic acid; 6 mim = 2-methylimidazolate; 7 eim = 2-ethylimidazolate; 8 pim = 2-propylimidazolate; 9 cim = imidazolate-2-carboxaldehyde; DMSO = dimethyl sulfoxide.
2. Synthesis Method
2.1. Facile Synthesis
2.2. Metal Sources
2.3. Upscale Synthesis
3. Structure Diversity of TIF-Ax
4. Special Properties of TIF-Ax
4.1. Solvent Stability
4.2. Guest Selectivity
4.3. Flexibility
5. Application
5.1. CO2 Separation
5.2. NH3 Adsorption
5.3. C2 Separation
5.4. CO2 Cycloaddition
5.5. Heavy Metal Adsorption
References
- Li, J.; Gao, Z.R.; Lin, Q.-F.; Liu, C.; Gao, F.; Lin, C.; Zhang, S.; Deng, H.; Mayoral, A.; Fan, W.; et al. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023, 379, 283–287.
- Su, Y.; Otake, K.-I.; Zheng, J.-J.; Horike, S.; Kitagawa, S.; Gu, C. Separating water isotopologues using diffusion-regulatory porous materials. Nature 2022, 611, 289–294.
- Tan, Y.-X.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal–organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144.
- Wan, C.P.; Yi, J.D.; Cao, R.; Huang, Y.B. Conductive Metal/Covalent Organic Frameworks for CO2 Electroreduction. Chin. J. Struct. Chem. 2022, 41, 2205001–2205014.
- Chen, R.; Chen, G.; He, Y.; Zhang, J. Coordination Assembly of Tetrahedral Ti4(embonate)6 Cages with Alkaline-Earth Metal Ions. Chin. J. Struct. Chem. 2022, 41, 2201001–2201006.
- Zhang, Y.; Liu, Y.; Wang, D.; Liu, J.; Zhao, J.; Chen, L. State-of-the-art advances in the syntheses, structures, and applications of polyoxometalate-based metal–organic frameworks. Polyoxometalates 2023, 2, 9140017.
- Dao, X.-Y.; Sun, W.-Y. Single- and mixed-metal-organic framework photocatalysts for carbon dioxide reduction. Inorg. Chem. Front. 2021, 8, 3178–3204.
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-Directed Strategy for Zeolite-Type Metal–Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559.
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943.
- Tian, Y.-Q.; Yao, S.-Y.; Gu, D.; Cui, K.-H.; Guo, D.-W.; Zhang, G.; Chen, Z.-X.; Zhao, D.-Y. Cadmium Imidazolate Frameworks with Polymorphism, High Thermal Stability, and a Large Surface Area. Chem.-A Eur. J. 2010, 16, 1137–1141.
- Zhang, J.-P.; Zhu, A.-X.; Lin, R.-B.; Qi, X.-L.; Chen, X.-M. Pore Surface Tailored SOD-Type Metal-Organic Zeolites. Adv. Mater. 2011, 23, 1268–1271.
- He, C.-T.; Jiang, L.; Ye, Z.-M.; Krishna, R.; Zhong, Z.-S.; Liao, P.-Q.; Xu, J.; Ouyang, G.; Zhang, J.-P.; Chen, X.-M. Exceptional Hydrophobicity of a Large-Pore Metal–Organic Zeolite. J. Am. Chem. Soc. 2015, 137, 7217–7223.
- Xu, T.; Zhou, B.; Tao, Y.; Shi, Z.; Jiang, W.; Abdellatief, M.; Cordova, K.E.; Zhang, Y.-B. Functionality-Induced Locking of Zeolitic Imidazolate Frameworks. Chem. Mater. 2023, 35, 490–498.
- Yang, J.; Zhang, Y.-B.; Liu, Q.; Trickett, C.A.; Gutiérrez-Puebla, E.; Monge, M.Á.; Cong, H.; Aldossary, A.; Deng, H.; Yaghi, O.M. Principles of Designing Extra-Large Pore Openings and Cages in Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2017, 139, 6448–6455.
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249.
- Li, M.-Y.; Liu, J.; Gao, R.; Lin, D.-Y.; Wang, F.; Zhang, J. Design and synthesis of zeolitic tetrazolate-imidazolate frameworks. Mater. Today Adv. 2021, 10, 100145.
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033.
- Wu, T.; Bu, X.; Zhang, J.; Feng, P. New Zeolitic Imidazolate Frameworks: From Unprecedented Assembly of Cubic Clusters to Ordered Cooperative Organization of Complementary Ligands. Chem. Mater. 2008, 20, 7377–7382.
- Wu, T.; Bu, X.; Liu, R.; Lin, Z.; Zhang, J.; Feng, P. A New Zeolitic Topology with Sixteen-Membered Ring and Multidimensional Large Pore Channels. Chem.-A Eur. J. 2008, 14, 7771–7773.
- Zheng, S.-T.; Li, Y.; Wu, T.; Nieto, R.A.; Feng, P.; Bu, X. Porous Lithium Imidazolate Frameworks Constructed with Charge-Complementary Ligands. Chem.-A Eur. J. 2010, 16, 13035–13040.
- Zheng, S.; Wu, T.; Zhang, J.; Chow, M.; Nieto, R.A.; Feng, P.; Bu, X. Porous Metal Carboxylate Boron Imidazolate Frameworks. Angew. Chem. Int. Ed. 2010, 49, 5362–5366.
- Wang, F.; Tan, Y.-X.; Yang, H.; Zhang, H.-X.; Kang, Y.; Zhang, J. A new approach towards tetrahedral imidazolate frameworks for high and selective CO2 uptake. Chem. Commun. 2011, 47, 5828–5830.
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359.
- Abdul Hamid, M.R.; Shean Yaw, T.C.; Mohd Tohir, M.Z.; Wan Abdul Karim Ghani, W.A.; Sutrisna, P.D.; Jeong, H.-K. Zeolitic imidazolate framework membranes for gas separations: Current state-of-the-art, challenges, and opportunities. J. Ind. Eng. Chem. 2021, 98, 17–41.
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 48968.
- Zanon, A.; Verpoort, F. Metals@ZIFs: Catalytic applications and size selective catalysis. Coord. Chem. Rev. 2017, 353, 201–222.
- Williams, K.; Meng, L.; Lee, S.; Lux, L.; Gao, W.; Ma, S. Imparting brønsted acidity into a zeolitic imidazole framework. Inorg. Chem. Front. 2016, 3, 393–396.
- Gao, C.; Mu, S.; Yan, R.; Chen, F.; Ma, T.; Cao, S.; Li, S.; Ma, L.; Wang, Y.; Cheng, C. Recent Advances in ZIF-Derived Atomic Metal–N–C Electrocatalysts for Oxygen Reduction Reaction: Synthetic Strategies, Active Centers, and Stabilities. Small 2022, 18, 2105409.
- Ahmad, R.; Khan, U.A.; Iqbal, N.; Noor, T. Zeolitic imidazolate framework (ZIF)-derived porous carbon materials for supercapacitors: An overview. RSC Adv. 2020, 10, 43733–43750.
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Abid, H.R.; Tadé, M.O.; Shao, Z. Advances in Zeolite Imidazolate Frameworks (ZIFs) Derived Bifunctional Oxygen Electrocatalysts and Their Application in Zinc–Air Batteries. Adv. Energy Mater. 2021, 11, 2100514.
- Song, X.; Jiang, Y.; Cheng, F.; Earnshaw, J.; Na, J.; Li, X.; Yamauchi, Y. Hollow Carbon-Based Nanoarchitectures Based on ZIF: Inward/Outward Contraction Mechanism and Beyond. Small 2021, 17, 2004142.
- Dutta, S.; Liu, Z.; Han, H.; Indra, A.; Song, T. Electrochemical Energy Conversion and Storage with Zeolitic Imidazolate Framework Derived Materials: A Perspective. ChemElectroChem 2018, 5, 3571–3588.
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent Development of Zeolitic Imidazolate Frameworks (ZIFs) Derived Porous Carbon Based Materials as Electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257.
- Yang, H.; Chen, X.; Chen, W.-T.; Wang, Q.; Cuello, N.C.; Nafady, A.; Al-Enizi, A.M.; Waterhouse, G.I.N.; Goenaga, G.A.; Zawodzinski, T.A.; et al. Tunable Synthesis of Hollow Metal–Nitrogen–Carbon Capsules for Efficient Oxygen Reduction Catalysis in Proton Exchange Membrane Fuel Cells. ACS Nano 2019, 13, 8087–8098.
- Hou, C.-C.; Xu, Q. Metal–Organic Frameworks for Energy. Adv. Energy Mater. 2019, 9, 1801307.
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448.
- Yang, Q.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. Pd Nanocubes@ZIF-8: Integration of Plasmon-Driven Photothermal Conversion with a Metal–Organic Framework for Efficient and Selective Catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689.
- Maleki, A.; Shahbazi, M.-A.; Alinezhad, V.; Santos, H.A. The Progress and Prospect of Zeolitic Imidazolate Frameworks in Cancer Therapy, Antibacterial Activity, and Biomineralization. Adv. Healthc. Mater. 2020, 9, 2000248.
- Zhao, Z.; Gao, Z.; Lan, D.; Kou, K. MOFs-derived hollow materials for electromagnetic wave absorption: Prospects and challenges. J. Mater. Sci. Mater. Electron. 2021, 32, 25631–25648.
- Hou, J.; Ashling, C.W.; Collins, S.M.; Krajnc, A.; Zhou, C.; Longley, L.; Johnstone, D.N.; Chater, P.A.; Li, S.; Coulet, M.-V.; et al. Metal-organic framework crystal-glass composites. Nat. Commun. 2019, 10, 2580.
- Madsen, R.S.K.; Qiao, A.; Sen, J.; Hung, I.; Chen, K.; Gan, Z.; Sen, S.; Yue, Y. Ultrahigh-field 67Zn NMR reveals short-range disorder in zeolitic imidazolate framework glasses. Science 2020, 367, 1473–1476.
- Ma, N.; Horike, S. Metal–Organic Network-Forming Glasses. Chem. Rev. 2022, 122, 4163–4203.
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H.-L. Metal–organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562–10611.
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326.
- Gao, S.; Hou, J.; Deng, Z.; Wang, T.; Beyer, S.; Buzanich, A.G.; Richardson, J.J.; Rawal, A.; Seidel, R.; Zulkifli, M.Y.; et al. Improving the Acidic Stability of Zeolitic Imidazolate Frameworks by Biofunctional Molecules. Chem 2019, 5, 1597–1608.
- Yang, X.-G.; Zhang, J.-R.; Tian, X.-K.; Qin, J.-H.; Zhang, X.-Y.; Ma, L.-F. Enhanced Activity of Enzyme Immobilized on Hydrophobic ZIF-8 Modified by Ni2+ Ions. Angew. Chem.-Int. Ed. 2023, 62, e202216699.
- Guo, S.; Li, H.-Z.; Wang, Z.-W.; Zhu, Z.-Y.; Zhang, S.-H.; Wang, F.; Zhang, J. Syntheses of new zeolitic imidazolate frameworks in dimethyl sulfoxide. Inorg. Chem. Front. 2022, 9, 2011–2015.
- Wang, H.; Pei, X.; Kalmutzki, M.J.; Yang, J.; Yaghi, O.M. Large Cages of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2022, 55, 707–721.
- Li, M.Y.; Wang, F.; Gu, Z.G.; Zhang, J. Synthesis of homochiral zeolitic metal-organic frameworks with amino acid and tetrazolates for chiral recognition. RSC Adv. 2017, 7, 4872–4875.
- Wang, F.; Hou, D.-C.; Yang, H.; Kang, Y.; Zhang, J. Tetrahedral tetrazolate frameworks for high CO2 and H2 uptake. Dalton Trans. 2014, 43, 3210–3214.
- Wang, F.; Fu, H.-R.; Kang, Y.; Zhang, J. A new approach towards zeolitic tetrazolate-imidazolate frameworks (ZTIFs) with uncoordinated N-heteroatom sites for high CO2 uptake. Chem. Commun. 2014, 50, 12065–12068.
- Zha, X.; Li, X.; Al-Omari, A.A.; Liu, S.; Liang, C.-C.; Al-Ghourani, A.A.; Abdellatief, M.; Yang, J.; Nguyen, H.L.; Al-Maythalony, B.; et al. Zeolite NPO-Type Azolate Frameworks. Angew. Chem. Int. Ed. 2022, 61, e202207467.
- Gai, Y.; Chen, X.; Yang, H.; Wang, Y.; Bu, X.; Feng, P. A new strategy for constructing a disulfide-functionalized ZIF-8 analogue using structure-directing ligand-ligand covalent interaction. Chem. Commun. 2018, 54, 12109–12112.
- Cui, P.; Ma, Y.G.; Li, H.H.; Zhao, B.; Li, J.R.; Cheng, P.; Balbuena, P.B.; Zhou, H.C. Multipoint interactions enhanced CO2 uptake: A zeolite-like zinc-tetrazole framework with 24-nuclear zinc cages. J. Am. Chem. Soc. 2012, 134, 18892–18895.
- Qin, J.S.; Du, D.Y.; Li, W.L.; Zhang, J.P.; Li, S.L.; Su, Z.M.; Wang, X.L.; Xu, Q.; Shao, K.Z.; Lan, Y.Q. N-rich zeolite-like metal-organic framework with sodalite topology: High CO2 uptake, selective gas adsorption and efficient drug delivery. Chem. Sci. 2012, 3, 2114–2118.
- Tang, Y.H.; Wang, F.; Liu, J.X.; Zhang, J. Diverse tetrahedral tetrazolate frameworks with N-rich surface. Chem. Commun. 2016, 52, 5625–5628.
- Wang, F.; Tang, Y.H.; Zhang, J. Achievement of Bulky Homochirality in Zeolitic Imidazolate-Related Frameworks. Inorg. Chem. 2015, 54, 11064–11066.
- Yang, E.; Li, H.-Y.; Wang, F.; Yang, H.; Zhang, J. Enhancing CO2 adsorption enthalpy and selectivity via amino functionalization of a tetrahedral framework material. CrystEngComm 2013, 15, 658–661.
- Ruan, M.; Li, A.; Wen, Y.; Zhou, L.; Zhang, J.; Xuan, X. Adenine-based bio-MOFs with high water and acid–base stability for ammonia capture. CrystEngComm 2022, 24, 7420–7426.
- Lou, B.; He, F. Coordination polymers as potential solid forms of drugs: Three zinc(ii) coordination polymers of theophylline with biocompatible organic acids. New J. Chem. 2013, 37, 309–316.
- Wang, F.; Kang, Y. Unusual cadmium(II)–adenine paddle-wheel units for the construction of a metal-organic framework with mog topology. Inorg. Chem. Commun. 2012, 20, 266–268.
- Guo, S.; Zhang, S.-H.; Wang, F.; Zhang, J. Syntheses of tetrahedral imidazolate frameworks with auxiliary ligand in DMSO. J. Solid State Chem. 2022, 311, 123101.
- Li, H.-Z.; Sun, Y.; Lin, D.; Yang, W.; Wang, F. Facile syntheses of tetrahedral imidazolate framework for CO2 separation. J. Solid State Chem. 2021, 297, 122100.
- Wang, F.; Yang, H.; Kang, Y.; Zhang, J. Guest selectivity of a porous tetrahedral imidazolate framework material during self-assembly. J. Mater. Chem. 2012, 22, 19732–19737.
- Li, H.-Z.; Li, Q.-H.; Yao, M.; Han, Y.-P.; Otake, K.-I.; Kitagawa, S.; Wang, F.; Zhang, J. Metal–Organic Framework with Structural Flexibility Responding Specifically to Acetylene and Its Adsorption Behavior. ACS Appl. Mater. Interfaces 2022, 14, 45451–45457.
- Ding, Q.; Zhang, Z.; Liu, Y.; Chai, K.; Krishna, R.; Zhang, S. One-Step Ethylene Purification from Ternary Mixtures in a Metal–Organic Framework with Customized Pore Chemistry and Shape. Angew. Chem. Int. Ed. 2022, 61, e202208134.
- Wang, J.-X.; Li, H.-G.; Ye, S.-S.; Zhang, J.-B.; Chen, B.-H. Halogen-rich zinc-adeninate framework construction and its catalytic performance on CO2 cycloaddition without cocatalyst. CIESC J. 2021, 72, 3686–3695.
- Ma, Y.; You, D.; Fang, Y.; Luo, J.; Pan, Q.; Liu, Y.; Wang, F.; Yang, W. Confined growth of MOF in chitosan matrix for removal of trace Pb(Ⅱ) from reclaimed water. Sep. Purif. Technol. 2022, 294, 121223.




