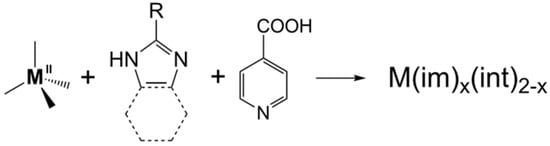
Zeolitic imidazolate frameworks (ZIFs) are an important subclass of metal–organic frameworks (MOFs). A new kind of MOF, namely tetrahedral imidazolate frameworks with auxiliary ligands (TIF-Ax) was reported, by adding linear ligands (Hint) into the zinc–imidazolate system. Introducing linear ligands into the M2+-imidazolate system overcomes the limitation of imidazole derivatives. Thanks to the synergistic effect of two different types of ligands, a series of new TIF-Ax with interesting topologies and a special pore environment has been reported, and they have attracted extensive attention in gas adsorption, separation, catalysis, heavy metal ion capture, and so on.
- zeolite
- metal–organic frameworks
- zeolitic imidazolate frameworks
- tetrahedral imidazolate frameworks
- TIF-A1
- adsorption and separation
1. Introduction
|
Name |
Formula |
Space Group |
Topology |
Ref. |
|---|---|---|---|---|
|
TIF-A1 |
[Zn(ad)(int)](DMF) 1 |
Pna21 |
dmp |
[22] |
|
TIF-A2 |
Zn2(im)3(int) 2 |
Pca21 |
dia |
[22] |
|
TIF-A3 |
Zn2(im)(int)2(OH) |
C2/c |
neb |
[22] |
|
2-NH2-TIF-A1 |
[Zn(ad)(2-NH2-int)](DMF) |
Pna21 |
dmp |
[58] |
|
3-NH2-TIF-A1 |
[Zn(3-NH2-int)(ad)](DMF) [Zn(3-NH2-int)(ad)](DMA) 3 |
Pna21 |
dmp |
[59] |
|
Zn-thp-nit |
[Zn(thp)(nit)] 4 |
Pbca |
— |
[60] |
|
Cd-ad-int |
[Cd2(ad)2(int)2(DMF) (H2O)](DMF) |
P21/n |
mog |
[61] |
|
TIF-A4 |
Zn(im)(Ac) 5 |
Ima2 |
dia |
[62] |
|
TIF-A5 |
Zn(2-mim)(Ac) 6 |
P21/c |
sql |
[62] |
|
TIF-A6 |
Zn(2-eim)(Ac) 7 |
P21/c |
sql |
[62] |
|
TIF-A7 |
Zn(2-pim)(Ac) 8 |
Pna21 |
sql |
[62] |
|
TIF-A8 |
[Zn2(OH-)(Ac)(2-cim)2](DMSO) 9 |
Cmc21 |
sql |
[62] |
1 ad = adeninate; int = isonicotinate; DMF = N,N′-dimethylformamide; 2 im = imidazolate; 3 DMA = N,N′-dimethylacetamide; 4 thp = theophylline; nit = nicotinic acid; 5 Ac = acetic acid; 6 mim = 2-methylimidazolate; 7 eim = 2-ethylimidazolate; 8 pim = 2-propylimidazolate; 9 cim = imidazolate-2-carboxaldehyde; DMSO = dimethyl sulfoxide.
2. Synthesis Method
2.1. Facile Synthesis
2.2. Metal Sources
2.3. Upscale Synthesis
3. Structure Diversity of TIF-Ax
4. Special Properties of TIF-Ax
4.1. Solvent Stability
4.2. Guest Selectivity
4.3. Flexibility
5. Application
5.1. CO2 Separation
5.2. NH3 Adsorption
5.3. C2 Separation
5.4. CO2 Cycloaddition
5.5. Heavy Metal Adsorption
This entry is adapted from the peer-reviewed paper 10.3390/molecules28166031
References
- Li, J.; Gao, Z.R.; Lin, Q.-F.; Liu, C.; Gao, F.; Lin, C.; Zhang, S.; Deng, H.; Mayoral, A.; Fan, W.; et al. A 3D extra-large-pore zeolite enabled by 1D-to-3D topotactic condensation of a chain silicate. Science 2023, 379, 283–287.
- Su, Y.; Otake, K.-I.; Zheng, J.-J.; Horike, S.; Kitagawa, S.; Gu, C. Separating water isotopologues using diffusion-regulatory porous materials. Nature 2022, 611, 289–294.
- Tan, Y.-X.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal–organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144.
- Wan, C.P.; Yi, J.D.; Cao, R.; Huang, Y.B. Conductive Metal/Covalent Organic Frameworks for CO2 Electroreduction. Chin. J. Struct. Chem. 2022, 41, 2205001–2205014.
- Chen, R.; Chen, G.; He, Y.; Zhang, J. Coordination Assembly of Tetrahedral Ti4(embonate)6 Cages with Alkaline-Earth Metal Ions. Chin. J. Struct. Chem. 2022, 41, 2201001–2201006.
- Zhang, Y.; Liu, Y.; Wang, D.; Liu, J.; Zhao, J.; Chen, L. State-of-the-art advances in the syntheses, structures, and applications of polyoxometalate-based metal–organic frameworks. Polyoxometalates 2023, 2, 9140017.
- Dao, X.-Y.; Sun, W.-Y. Single- and mixed-metal-organic framework photocatalysts for carbon dioxide reduction. Inorg. Chem. Front. 2021, 8, 3178–3204.
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-Directed Strategy for Zeolite-Type Metal–Organic Frameworks: Zinc(II) Imidazolates with Unusual Zeolitic Topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559.
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943.
- Tian, Y.-Q.; Yao, S.-Y.; Gu, D.; Cui, K.-H.; Guo, D.-W.; Zhang, G.; Chen, Z.-X.; Zhao, D.-Y. Cadmium Imidazolate Frameworks with Polymorphism, High Thermal Stability, and a Large Surface Area. Chem.-A Eur. J. 2010, 16, 1137–1141.
- Zhang, J.-P.; Zhu, A.-X.; Lin, R.-B.; Qi, X.-L.; Chen, X.-M. Pore Surface Tailored SOD-Type Metal-Organic Zeolites. Adv. Mater. 2011, 23, 1268–1271.
- He, C.-T.; Jiang, L.; Ye, Z.-M.; Krishna, R.; Zhong, Z.-S.; Liao, P.-Q.; Xu, J.; Ouyang, G.; Zhang, J.-P.; Chen, X.-M. Exceptional Hydrophobicity of a Large-Pore Metal–Organic Zeolite. J. Am. Chem. Soc. 2015, 137, 7217–7223.
- Xu, T.; Zhou, B.; Tao, Y.; Shi, Z.; Jiang, W.; Abdellatief, M.; Cordova, K.E.; Zhang, Y.-B. Functionality-Induced Locking of Zeolitic Imidazolate Frameworks. Chem. Mater. 2023, 35, 490–498.
- Yang, J.; Zhang, Y.-B.; Liu, Q.; Trickett, C.A.; Gutiérrez-Puebla, E.; Monge, M.Á.; Cong, H.; Aldossary, A.; Deng, H.; Yaghi, O.M. Principles of Designing Extra-Large Pore Openings and Cages in Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2017, 139, 6448–6455.
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249.
- Li, M.-Y.; Liu, J.; Gao, R.; Lin, D.-Y.; Wang, F.; Zhang, J. Design and synthesis of zeolitic tetrazolate-imidazolate frameworks. Mater. Today Adv. 2021, 10, 100145.
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033.
- Wu, T.; Bu, X.; Zhang, J.; Feng, P. New Zeolitic Imidazolate Frameworks: From Unprecedented Assembly of Cubic Clusters to Ordered Cooperative Organization of Complementary Ligands. Chem. Mater. 2008, 20, 7377–7382.
- Wu, T.; Bu, X.; Liu, R.; Lin, Z.; Zhang, J.; Feng, P. A New Zeolitic Topology with Sixteen-Membered Ring and Multidimensional Large Pore Channels. Chem.-A Eur. J. 2008, 14, 7771–7773.
- Zheng, S.-T.; Li, Y.; Wu, T.; Nieto, R.A.; Feng, P.; Bu, X. Porous Lithium Imidazolate Frameworks Constructed with Charge-Complementary Ligands. Chem.-A Eur. J. 2010, 16, 13035–13040.
- Zheng, S.; Wu, T.; Zhang, J.; Chow, M.; Nieto, R.A.; Feng, P.; Bu, X. Porous Metal Carboxylate Boron Imidazolate Frameworks. Angew. Chem. Int. Ed. 2010, 49, 5362–5366.
- Wang, F.; Tan, Y.-X.; Yang, H.; Zhang, H.-X.; Kang, Y.; Zhang, J. A new approach towards tetrahedral imidazolate frameworks for high and selective CO2 uptake. Chem. Commun. 2011, 47, 5828–5830.
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359.
- Abdul Hamid, M.R.; Shean Yaw, T.C.; Mohd Tohir, M.Z.; Wan Abdul Karim Ghani, W.A.; Sutrisna, P.D.; Jeong, H.-K. Zeolitic imidazolate framework membranes for gas separations: Current state-of-the-art, challenges, and opportunities. J. Ind. Eng. Chem. 2021, 98, 17–41.
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137, 48968.
- Zanon, A.; Verpoort, F. Metals@ZIFs: Catalytic applications and size selective catalysis. Coord. Chem. Rev. 2017, 353, 201–222.
- Williams, K.; Meng, L.; Lee, S.; Lux, L.; Gao, W.; Ma, S. Imparting brønsted acidity into a zeolitic imidazole framework. Inorg. Chem. Front. 2016, 3, 393–396.
- Gao, C.; Mu, S.; Yan, R.; Chen, F.; Ma, T.; Cao, S.; Li, S.; Ma, L.; Wang, Y.; Cheng, C. Recent Advances in ZIF-Derived Atomic Metal–N–C Electrocatalysts for Oxygen Reduction Reaction: Synthetic Strategies, Active Centers, and Stabilities. Small 2022, 18, 2105409.
- Ahmad, R.; Khan, U.A.; Iqbal, N.; Noor, T. Zeolitic imidazolate framework (ZIF)-derived porous carbon materials for supercapacitors: An overview. RSC Adv. 2020, 10, 43733–43750.
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Abid, H.R.; Tadé, M.O.; Shao, Z. Advances in Zeolite Imidazolate Frameworks (ZIFs) Derived Bifunctional Oxygen Electrocatalysts and Their Application in Zinc–Air Batteries. Adv. Energy Mater. 2021, 11, 2100514.
- Song, X.; Jiang, Y.; Cheng, F.; Earnshaw, J.; Na, J.; Li, X.; Yamauchi, Y. Hollow Carbon-Based Nanoarchitectures Based on ZIF: Inward/Outward Contraction Mechanism and Beyond. Small 2021, 17, 2004142.
- Dutta, S.; Liu, Z.; Han, H.; Indra, A.; Song, T. Electrochemical Energy Conversion and Storage with Zeolitic Imidazolate Framework Derived Materials: A Perspective. ChemElectroChem 2018, 5, 3571–3588.
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent Development of Zeolitic Imidazolate Frameworks (ZIFs) Derived Porous Carbon Based Materials as Electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257.
- Yang, H.; Chen, X.; Chen, W.-T.; Wang, Q.; Cuello, N.C.; Nafady, A.; Al-Enizi, A.M.; Waterhouse, G.I.N.; Goenaga, G.A.; Zawodzinski, T.A.; et al. Tunable Synthesis of Hollow Metal–Nitrogen–Carbon Capsules for Efficient Oxygen Reduction Catalysis in Proton Exchange Membrane Fuel Cells. ACS Nano 2019, 13, 8087–8098.
- Hou, C.-C.; Xu, Q. Metal–Organic Frameworks for Energy. Adv. Energy Mater. 2019, 9, 1801307.
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414–1448.
- Yang, Q.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. Pd Nanocubes@ZIF-8: Integration of Plasmon-Driven Photothermal Conversion with a Metal–Organic Framework for Efficient and Selective Catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689.
- Maleki, A.; Shahbazi, M.-A.; Alinezhad, V.; Santos, H.A. The Progress and Prospect of Zeolitic Imidazolate Frameworks in Cancer Therapy, Antibacterial Activity, and Biomineralization. Adv. Healthc. Mater. 2020, 9, 2000248.
- Zhao, Z.; Gao, Z.; Lan, D.; Kou, K. MOFs-derived hollow materials for electromagnetic wave absorption: Prospects and challenges. J. Mater. Sci. Mater. Electron. 2021, 32, 25631–25648.
- Hou, J.; Ashling, C.W.; Collins, S.M.; Krajnc, A.; Zhou, C.; Longley, L.; Johnstone, D.N.; Chater, P.A.; Li, S.; Coulet, M.-V.; et al. Metal-organic framework crystal-glass composites. Nat. Commun. 2019, 10, 2580.
- Madsen, R.S.K.; Qiao, A.; Sen, J.; Hung, I.; Chen, K.; Gan, Z.; Sen, S.; Yue, Y. Ultrahigh-field 67Zn NMR reveals short-range disorder in zeolitic imidazolate framework glasses. Science 2020, 367, 1473–1476.
- Ma, N.; Horike, S. Metal–Organic Network-Forming Glasses. Chem. Rev. 2022, 122, 4163–4203.
- Fonseca, J.; Gong, T.; Jiao, L.; Jiang, H.-L. Metal–organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562–10611.
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326.
- Gao, S.; Hou, J.; Deng, Z.; Wang, T.; Beyer, S.; Buzanich, A.G.; Richardson, J.J.; Rawal, A.; Seidel, R.; Zulkifli, M.Y.; et al. Improving the Acidic Stability of Zeolitic Imidazolate Frameworks by Biofunctional Molecules. Chem 2019, 5, 1597–1608.
- Yang, X.-G.; Zhang, J.-R.; Tian, X.-K.; Qin, J.-H.; Zhang, X.-Y.; Ma, L.-F. Enhanced Activity of Enzyme Immobilized on Hydrophobic ZIF-8 Modified by Ni2+ Ions. Angew. Chem.-Int. Ed. 2023, 62, e202216699.
- Guo, S.; Li, H.-Z.; Wang, Z.-W.; Zhu, Z.-Y.; Zhang, S.-H.; Wang, F.; Zhang, J. Syntheses of new zeolitic imidazolate frameworks in dimethyl sulfoxide. Inorg. Chem. Front. 2022, 9, 2011–2015.
- Wang, H.; Pei, X.; Kalmutzki, M.J.; Yang, J.; Yaghi, O.M. Large Cages of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2022, 55, 707–721.
- Li, M.Y.; Wang, F.; Gu, Z.G.; Zhang, J. Synthesis of homochiral zeolitic metal-organic frameworks with amino acid and tetrazolates for chiral recognition. RSC Adv. 2017, 7, 4872–4875.
- Wang, F.; Hou, D.-C.; Yang, H.; Kang, Y.; Zhang, J. Tetrahedral tetrazolate frameworks for high CO2 and H2 uptake. Dalton Trans. 2014, 43, 3210–3214.
- Wang, F.; Fu, H.-R.; Kang, Y.; Zhang, J. A new approach towards zeolitic tetrazolate-imidazolate frameworks (ZTIFs) with uncoordinated N-heteroatom sites for high CO2 uptake. Chem. Commun. 2014, 50, 12065–12068.
- Zha, X.; Li, X.; Al-Omari, A.A.; Liu, S.; Liang, C.-C.; Al-Ghourani, A.A.; Abdellatief, M.; Yang, J.; Nguyen, H.L.; Al-Maythalony, B.; et al. Zeolite NPO-Type Azolate Frameworks. Angew. Chem. Int. Ed. 2022, 61, e202207467.
- Gai, Y.; Chen, X.; Yang, H.; Wang, Y.; Bu, X.; Feng, P. A new strategy for constructing a disulfide-functionalized ZIF-8 analogue using structure-directing ligand-ligand covalent interaction. Chem. Commun. 2018, 54, 12109–12112.
- Cui, P.; Ma, Y.G.; Li, H.H.; Zhao, B.; Li, J.R.; Cheng, P.; Balbuena, P.B.; Zhou, H.C. Multipoint interactions enhanced CO2 uptake: A zeolite-like zinc-tetrazole framework with 24-nuclear zinc cages. J. Am. Chem. Soc. 2012, 134, 18892–18895.
- Qin, J.S.; Du, D.Y.; Li, W.L.; Zhang, J.P.; Li, S.L.; Su, Z.M.; Wang, X.L.; Xu, Q.; Shao, K.Z.; Lan, Y.Q. N-rich zeolite-like metal-organic framework with sodalite topology: High CO2 uptake, selective gas adsorption and efficient drug delivery. Chem. Sci. 2012, 3, 2114–2118.
- Tang, Y.H.; Wang, F.; Liu, J.X.; Zhang, J. Diverse tetrahedral tetrazolate frameworks with N-rich surface. Chem. Commun. 2016, 52, 5625–5628.
- Wang, F.; Tang, Y.H.; Zhang, J. Achievement of Bulky Homochirality in Zeolitic Imidazolate-Related Frameworks. Inorg. Chem. 2015, 54, 11064–11066.
- Yang, E.; Li, H.-Y.; Wang, F.; Yang, H.; Zhang, J. Enhancing CO2 adsorption enthalpy and selectivity via amino functionalization of a tetrahedral framework material. CrystEngComm 2013, 15, 658–661.
- Ruan, M.; Li, A.; Wen, Y.; Zhou, L.; Zhang, J.; Xuan, X. Adenine-based bio-MOFs with high water and acid–base stability for ammonia capture. CrystEngComm 2022, 24, 7420–7426.
- Lou, B.; He, F. Coordination polymers as potential solid forms of drugs: Three zinc(ii) coordination polymers of theophylline with biocompatible organic acids. New J. Chem. 2013, 37, 309–316.
- Wang, F.; Kang, Y. Unusual cadmium(II)–adenine paddle-wheel units for the construction of a metal-organic framework with mog topology. Inorg. Chem. Commun. 2012, 20, 266–268.
- Guo, S.; Zhang, S.-H.; Wang, F.; Zhang, J. Syntheses of tetrahedral imidazolate frameworks with auxiliary ligand in DMSO. J. Solid State Chem. 2022, 311, 123101.
- Li, H.-Z.; Sun, Y.; Lin, D.; Yang, W.; Wang, F. Facile syntheses of tetrahedral imidazolate framework for CO2 separation. J. Solid State Chem. 2021, 297, 122100.
- Wang, F.; Yang, H.; Kang, Y.; Zhang, J. Guest selectivity of a porous tetrahedral imidazolate framework material during self-assembly. J. Mater. Chem. 2012, 22, 19732–19737.
- Li, H.-Z.; Li, Q.-H.; Yao, M.; Han, Y.-P.; Otake, K.-I.; Kitagawa, S.; Wang, F.; Zhang, J. Metal–Organic Framework with Structural Flexibility Responding Specifically to Acetylene and Its Adsorption Behavior. ACS Appl. Mater. Interfaces 2022, 14, 45451–45457.
- Ding, Q.; Zhang, Z.; Liu, Y.; Chai, K.; Krishna, R.; Zhang, S. One-Step Ethylene Purification from Ternary Mixtures in a Metal–Organic Framework with Customized Pore Chemistry and Shape. Angew. Chem. Int. Ed. 2022, 61, e202208134.
- Wang, J.-X.; Li, H.-G.; Ye, S.-S.; Zhang, J.-B.; Chen, B.-H. Halogen-rich zinc-adeninate framework construction and its catalytic performance on CO2 cycloaddition without cocatalyst. CIESC J. 2021, 72, 3686–3695.
- Ma, Y.; You, D.; Fang, Y.; Luo, J.; Pan, Q.; Liu, Y.; Wang, F.; Yang, W. Confined growth of MOF in chitosan matrix for removal of trace Pb(Ⅱ) from reclaimed water. Sep. Purif. Technol. 2022, 294, 121223.
