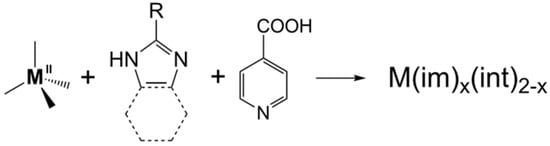
Zeolitic imidazolate frameworks (ZIFs) are an important subclass of metal–organic frameworks (MOFs). A new kind of MOF, namely tetrahedral imidazolate frameworks with auxiliary ligands (TIF-Ax) was reported, by adding linear ligands (Hint) into the zinc–imidazolate system. Introducing linear ligands into the M2+-imidazolate system overcomes the limitation of imidazole derivatives. Thanks to the synergistic effect of two different types of ligands, a series of new TIF-Ax with interesting topologies and a special pore environment has been reported, and they have attracted extensive attention in gas adsorption, separation, catalysis, heavy metal ion capture, and so on.
- zeolite
- metal–organic frameworks
- zeolitic imidazolate frameworks
- tetrahedral imidazolate frameworks
- TIF-A1
- adsorption and separation
1. Introduction
|
Name |
Formula |
Space Group |
Topology |
Ref. |
|---|---|---|---|---|
|
TIF-A1 |
[Zn(ad)(int)](DMF) 1 |
Pna21 |
dmp |
[22] |
|
TIF-A2 |
Zn2(im)3(int) 2 |
Pca21 |
dia |
[22] |
|
TIF-A3 |
Zn2(im)(int)2(OH) |
C2/c |
neb |
[22] |
|
2-NH2-TIF-A1 |
[Zn(ad)(2-NH2-int)](DMF) |
Pna21 |
dmp |
|
|
3-NH2-TIF-A1 |
[Zn(3-NH2-int)(ad)](DMF) [Zn(3-NH2-int)(ad)](DMA) 3 |
Pna21 |
dmp |
|
|
Zn-thp-nit |
[Zn(thp)(nit)] 4 |
Pbca |
— |
|
|
Cd-ad-int |
[Cd2(ad)2(int)2(DMF) (H2O)](DMF) |
P21/n |
mog |
|
|
TIF-A4 |
Zn(im)(Ac) 5 |
Ima2 |
dia |
|
|
TIF-A5 |
Zn(2-mim)(Ac) 6 |
P21/c |
sql |
|
|
TIF-A6 |
Zn(2-eim)(Ac) 7 |
P21/c |
sql |
|
|
TIF-A7 |
Zn(2-pim)(Ac) 8 |
Pna21 |
sql |
|
|
TIF-A8 |
[Zn2(OH-)(Ac)(2-cim)2](DMSO) 9 |
Cmc21 |
sql |
1 ad = adeninate; int = isonicotinate; DMF = N,N′-dimethylformamide; 2 im = imidazolate; 3 DMA = N,N′-dimethylacetamide; 4 thp = theophylline; nit = nicotinic acid; 5 Ac = acetic acid; 6 mim = 2-methylimidazolate; 7 eim = 2-ethylimidazolate; 8 pim = 2-propylimidazolate; 9 cim = imidazolate-2-carboxaldehyde; DMSO = dimethyl sulfoxide.
2. Synthesis Method
2.1. Facile Synthesis
2.2. Metal Sources
Inspired by the aforementioned outcomes achieved through stirring and heating at 120 °C in a brief timeframe, and mindful of the potential for the reaction between nitrate and carboxylic acid to generate corrosive nitric acid, which might compromise the equipment’s integrity and influence the stability of the final MOFs, alternative metal sources were employed to investigate the feasibility of substituting Zn(NO3)2∙6H2O. Similarly, the yields of TIF-A1 synthesized from ZnO and Zn(CH3COO)2∙2H2O were 75.5% and 80.5%, respectively [63][67]. This shows that the metal source has good substitutability.2.3. Upscale Synthesis
The potential for synthesizing a kilogram of TIF-A1 was further investigated using the aforementioned methods. Interestingly, through the scaling up of reactant quantities, a yield of over 800 g of TIF-A1 was achieved in a single heating process at 120 °C [63][67]. The resulting samples exhibited a similar crystallinity, morphology, and BET surface area to those obtained through the original solvothermal method.3. Structure Diversity of TIF-Ax
TIF-A1 is a 4-connected metal–organic framework with Zn2+ as the metal center, adenine (ad) as the main ligand, and isonicotinic acid (Hint) as the auxiliary ligand. Both ligands bind to Zn2+ via N and carboxyl O on heterocycles. It was observed that TIF-A1~A3 has a different topology, which was regulated by changing the type of imidazole salts in the framework. 2-NH2-TIF-A1 and 3-NH2-TIF-A1 were prepared by functional modification, and their functional groups could effectively adjust the pore environment and pore size. Cd-ad-int (int= isonicotinic acid) changed the metal center to have different building units, thus adjusting the structural network. Zn-thp-nit (thp = theophylline, nit = nicotinic acid) changed the isonicotinic acid ligand into nicotinic acid, which changed the angle of connection between the ligand and the metal. In TIF-A4 ~ A8, monocarboxylic acid with a coordination angle of ca. 120° was selected to replace the Hint, and a dia topological structure and layered structure were obtained.4. Special Properties of TIF-Ax
4.1. Solvent Stability
To realize the wide application of MOFs, they should first have good stability in harsh conditions, such as high temperature, high pressure, and acid and alkali environments. Interestingly, TIF-A1 can be stable in various organic solvents, such as water, N,N′-dimethylformamide (DMF), N,N′-diethylformamide (DEF), acetonitrile (CH3CN), ethyl acetate, toluene, n-hexane, dichloromethane (DCM), chloroform, methanol (MeOH), ethanol (EtOH), isopropanol (IPA), and n-butanol (n-BuOH). Furthermore, TIF-A1 can also maintain the framework after being soaked in a solution of pH= 2–1 [63][67]. The exceptional stability of TIF-A1 makes it a promising candidate for further industrial applications.4.2. Guest Selectivity
It is well known that solvent plays an important role in the structural variety of MOFs. Different solvent systems tend to produce different MOFs. However, it was found that the construction of TIF-A1 was independent of the solvents. TIF-A1 can accommodate amide solvents, including N,N′-dimethylformamide (DMF), ethyl urea (e-urea), N-methyl-1,2-pyrrolidone (NMP), and N,N′-dimethylacetamide (DMA). Interestingly, the crystallization process of TIF-A1 exhibited a strict selective trapping ability for these four solvent guests, and its selective order was DMF > e-urea > NMP > DMA (Scheme 2). This order of selection may be related to the size of the guest and the interaction between the host and guest. When the size of the guest is relatively small, the size of the guest is the main factor affecting the selection of guest molecules for TIF-A1. The four solvent guests are arranged in that order of molecular size: DMF < DMA < e-urea < NMP. DMF is very suitable to exist in the pores of TIF-A1. When the size of the guest is relatively large, TIF-A1 needs more energy to expand the pore to accommodate them. However, under this condition, the interaction between the host and the guest is dominant, and the energy needed to expand the pore will be partially offset. There is a strong N-H∙∙∙N hydrogen bond between e-urea and TIF-A1. There are non-classic C-H∙∙∙O and C-H∙∙∙N hydrogen bonds between NMP and TIF-A1. There is only a non-classic C-H∙∙∙O hydrogen bond between DMA and TIF-A1. Therefore, the selection order for TIF-A1 is e-urea > NMP > DMA. However, if the molecule is too large, it still depends mainly on the size of the guest. For example, TIF-A1 cannot trap 1,3-dimethyl-2-imidazolidinone (DMI) or N,N′-dimethylpropyleneurea (DMPU) [64][68].