Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liyu He | -- | 123 | 2023-08-31 09:02:12 | | | |
| 2 | Liyu He | + 2225 word(s) | 2348 | 2023-08-31 09:19:12 | | | | |
| 3 | Wendy Huang | -40 word(s) | 2308 | 2023-08-31 12:44:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, H.; Dai, W.; Xiao, L.; Sun, L.; He, L. Biopolymers and Their Applications in Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/48673 (accessed on 07 February 2026).
Li H, Dai W, Xiao L, Sun L, He L. Biopolymers and Their Applications in Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/48673. Accessed February 07, 2026.
Li, Hao, Wenni Dai, Li Xiao, Lin Sun, Liyu He. "Biopolymers and Their Applications in Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/48673 (accessed February 07, 2026).
Li, H., Dai, W., Xiao, L., Sun, L., & He, L. (2023, August 31). Biopolymers and Their Applications in Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/48673
Li, Hao, et al. "Biopolymers and Their Applications in Kidney Disease." Encyclopedia. Web. 31 August, 2023.
Copy Citation
Kidney disease has become a serious public health problem throughout the world, and its treatment and management constitute a huge global economic burden. Currently, the main clinical treatments are not sufficient to cure kidney diseases. During its development, nanotechnology has shown unprecedented potential for application to kidney diseases. However, nanotechnology has disadvantages such as high cost and poor bioavailability. In contrast, biopolymers are not only widely available but also highly bioavailable. Therefore, biopolymer-based nanosystems offer new promising solutions for the treatment of kidney diseases.
nanoparticles
biopolymer
drug delivery systems
kidney
chitosan
cellulose
alginate
1. Introduction
Nanotechnology is an emerging technology of the twenty-first century. The application of nanoparticles in treating and diagnosing diseases is an essential area of current nanotechnology research, known as nanomedicine [1]. Nanoparticles are particles with a size of 1–100 nm, including inorganic nanoparticles, lipid nanoparticles, carbon-based nanoparticles, polymer nanoparticles, and biomimetic nanoparticles [2]. There are many methods for the preparation of nanoparticles, which differ based on nanoparticle type. The common preparation methods include desolvation, thin-film hydration, microemulsion, covalent crosslinking, solvent evaporation, and so on [3][4][5]. Nanoparticles used as drug delivery systems can enhance the stability and solubility of drugs, promote drug transport across cell membranes, and prolong their circulation time to boost their safety and effectiveness. Additionally, nanoparticles can help to concentrate drugs at the site of disease or injury, while minimizing their adverse effects [6]. To date, various nanoparticles have been used as carriers for kidney-targeted drug delivery, making important contributions to, and showing great potential for the treatment of renal diseases.
Biopolymers are polymers produced by living organisms. Biopolymers have better biocompatibility, bioavailability, and bioreactivity than synthetic polymers [7]. These advantages have led to a wide range of applications in the biomedical field. The combination of nanotechnology and biopolymers in biopolymer-based nanosystems shows great potential for targeted drug delivery [8][9]. The biopolymer-based nanosystems are particularly promising for the treatment of kidney diseases with targeted drug carriers.
2. Chitosan
Chitosan is a highly abundant natural biopolymer derived from the exoskeletons of crustaceans such as lobsters and crabs, insects, and fungal cell walls [10]. Chitosan is a polysaccharide formed through the deacetylation of chitin, and its chemical structure consists of N-acetylglucosamine and D-glucosamine monomers (Figure 1). Chitosan’s molecular formula is C6H11NO4X2. Chitosan is formed after the N-deacetylation of chitin in reaction with a concentrated NaOH solution [11]. After deacetylation, as compared to chitin, chitosan acquires a higher water solubility and better chemical potential due to the free amino group [12]. The amino group on the surface of chitosan results in its positive charge [7]. This is of great interest for drug delivery to the kidney, as positively charged substances are more easily transported across the glomerular filtration barrier [13]. With continuous technological progress, the third generation of chitosan was introduced in the form of chitosan oligosaccharides, and chitosan became a designable bioactive compound, which greatly enhanced its application prospects [14]. At present, chitosan has a wide range of applications, including not only antibacterial, antitumor, and antioxidant applications [15][16][17] but also drug delivery, especially in its nanoform [18][19][20]. Chitosan nanoparticles are easy to produce, have a low toxicity, and are highly stable, biocompatible, and biodegradable. In addition, chitosan nanoparticles can release their encapsulated chemicals in a controlled manner [21]. As previously mentioned, chitosan with a low molecular weight can be selectively absorbed by megalin receptors in the kidney. Consequently, both chitosan nanoparticles and hybridized nanoparticles containing chitosan are effectively and selectively distributed in the kidney. All these advantages make chitosan nanoparticles an excellent vehicle for drug delivery to the kidney.

Figure 1. The chemical structure of chitosan and its origin.
Chitosan nanoparticles have important applications not only in hemorrhagic cystitis but also in the removal of harmful reactive oxygen species (ROS) from the kidney. M. Ahmad et al. [22] synthesized chitosan and curcumin composite nanoparticles through an ionic crosslinking method using glutaraldehyde as the crosslinking agent. They demonstrated that chitosan nanoparticles can have a strong effect as a biosorbent for the removal of ions such as cadmium or copper from the solution. The researchers prepared nanoparticles with particle sizes in the range of 2–40 nm, with good physical and chemical stability and easy dispersion in water. Curcumin, a well-known natural antioxidant, has a good ameliorating effect on oxidative stress induced by the toxic metal cadmium in the kidney when combined with chitosan nanoparticles. Similarly, M. Pang et al. [23] used low-molecular-weight chitosan to encapsulate lutein and celastrol in order to prepare nanomicelles that could be delivered to the kidney for the treatment of acute kidney injury (AKI). Nano micelles measuring 75.0 ± 5.0 nm in size were created with a consistent spherical shape, a zeta potential of 20.0 ± 3.2 mV, and a polymer dispersity index (PDI) of 0.13 ± 0.05. They cleverly linked citraconic anhydride to low-molecular-weight chitosan through amide bonding, linked the lutein to citraconic anhydride through an esterification reaction, and finally added the lipid-soluble compound celastrol to the hydrophobic core of the micelles. Moreover, due to the presence of amide bonds in these nanomicelles, which can be broken under acidic conditions, these nanomicelles also possess PH sensitivity, which contributes to the rapid release of the drug in the acidic environment of lysosomes after uptake by the cells. The renal targeting of these nanomicelles is due to the fact that low-molecular-weight chitosan can be specifically taken up by megalin receptors in renal tubular epithelial cells, similar to the mechanism for the specific uptake of TQ-nanoparticles by the kidney, as mentioned previously [24]. The average particle size of the nanomicelles was 75.0 ± 5.0 nm, with a uniform spherical shape, PDI of 0.27 ± 0.04, and zeta potential of 20.0 ± 3.2 mV. The distribution of the fluorescently labeled nanomicelles in mice was observed via NIR imaging, and it was demonstrated that the nanomicelle group had a stronger renal fluorescence intensity compared to the control group and showed rapid accumulation in the kidney.
Nanozymes are a focal topic of current research. Nanozymes are nanomaterials with an enzyme-like activity that have effective catalytic activity and a specific mechanism for a given reaction [25]. Z. Liu et al. [26] designed an ultra-small nanoenzyme for the treatment of AKI, and they successfully synthesized the nanoenzyme with an average particle size of 2 nm using chitosan, ruthenium(III) chloride trihydrate, and acetic acid with a solvothermal method. In addition, the nanoenzyme possesses multienzyme-like activity (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)) and acts as an antioxidant to effectively scavenge ROS in kidney cells for the treatment of AKI. The excellent antioxidant properties, biostability, biocompatibility, and renal accumulation of this nanoenzyme make it promising for further applications in other kidney diseases caused by ROS, such as diabetic kidney disease.
3. Cellulose
Cellulose is by far the most abundant, most common, and renewable natural biopolymer, which is derived from wood, cotton, bacteria, and fungi [27][28]. Cellulose is a polysaccharide with a linear structure consisting of multiple D-glucose units linked by β-1,4 glycosidic bonds (Figure 2) [29]. The cellulose molecular formula is (C6H10O5)n. Cellulose has a highly biocompatible and biodegradable nature, making it non-toxic [30]. Although cellulose is insoluble in water, which may limit its application in drug delivery, a series of cellulose derivatives can be formed through specific chemical reactions to improve its water solubility [31]. There are crystalline and amorphous regions in cellulose, and nanocrystalline cellulose is pure cellulose in a crystalline form with nanoscale dimensions [32]. The preparation methods of nanocrystalline cellulose mainly include acid hydrolysis, esterification using concentrated organic acids, (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl(TEMPO)-mediated oxidation, microbial or enzymatic hydrolysis, and periodate oxidation [33]. Nanocrystalline cellulose is a rod-shaped particle with an excellent aspect ratio, with a width of approximately 5–30 nm and a length of approximately 100–500 nm [34]. It also has superior axial Young’s modulus, high specific surface area, high transparency, and a number of other excellent properties, References [35][36]. As a result, nanocrystalline cellulose is widely used not only in drug delivery but also in many fields, such as papermaking, food production, and electronics [37][38][39]. In the kidney, nanocrystalline cellulose with a high aspect ratio (>10) is aligned with blood flow, filtered through the glomerulus, and subsequently reabsorbed by the renal unit from the tubular border. Due to the pharmacokinetic profile of the delivery platform, drugs bound to nanocrystalline cellulose are delivered to renal tubular cells [40]. Therefore, nanocrystalline cellulose has tremendous potential for renal drug delivery.
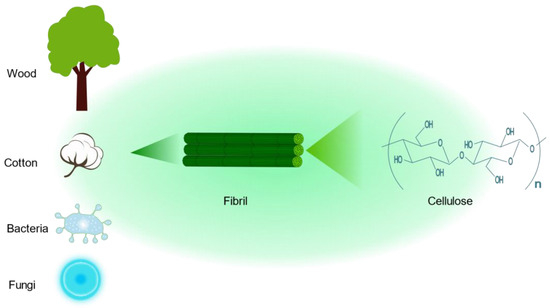
Figure 2. The chemical structure of cellulose and its sources.
Cellulose nanocrystals have a wide range of applications in drug delivery due to their chemical modifiability and biocompatibility, e.g., breast cancer, liver cancer, and myocardial diseases [41][42][43]. Hyperphosphatemia is one of the major metabolic disorders in CKD, and serum phosphate concentration is positively correlated with mortality in patients with advanced CKD [44]. Currently, the treatment of hyperphosphatemia in CKD patients is mainly based on dialysis and oral phosphate binders. Recently, Qimeng Zhang et al. developed cationic cellulose nanocrystals for the treatment of hyperphosphatemia, which can greatly reduce the blood phosphate level [45]. Cationic cellulose nanocrystals have a much larger surface charge than ordinary unmodified cellulose nanocrystals, which enables them to better bind anions (such as phosphate particles). Cationic cellulose nanocrystals were obtained via the wet and semi-dry process incorporation of cationization agents (e.g., glycidyltrimethylammonium chloride) into alkaline-activated cellulose nanocrystals [46]. The researchers of the study designed cationic cellulose nanocrystals, which are rigid rod-shaped nanoparticles with a diameter of approximately 10 nm, a length of approximately 150 nm, a large specific surface area, and a zeta potential of 62.3 mv. The cationic cellulose nanocrystals relied on electrostatic attraction and ion-exchange reactions to adsorb phosphate ions, with a binding capacity of approximately 74.3 mg/g, which is significantly higher than that of the commonly used phosphate binding agents (e.g., calcium carbonate, with approximately 39 mg/g) [47]. The researchers used these cationic cellulose nanocrystals in mice with chronic renal failure, and the serum phosphorus level of the model mice rapidly decreased to the same level as that of normal mice. The cationic cellulose nanocrystals were able to treat hyperphosphatemia by binding phosphate in the intestinal tract and effectively improved the inflammatory infiltration of the kidneys. In a study conducted by Sam Wong et al., strategic modifications of the primary and secondary hydroxyl groups on cellulose nanocrystals were performed through the introduction of amine and iodine substituents, respectively, were designed to serve as a potential drug delivery platform for the kidney [48]. The cellulose nanocrystals had a rod-like shape with a length of 164.0 ± 20.3 nm, a diameter of 10.52 ± 0.76 nm, and an aspect ratio of 15.97 ± 1.73 and exhibited good biocompatibility and safety, which allowed for their specific accumulation in the kidney, with an accumulation of 23% IA/g within 1 h after intravenous drug injection and the presence of 15% IA/g.
There are a large number of extensive studies on the synthesis, characterization, and biological interactions of cellulose nanocrystals, as well as the successful loading of various hydrophobic drugs. However, there are few studies on the use of these drugs for the treatment of renal diseases [49][50]. For example, curcumin, a polyphenolic compound with antioxidant properties, can be incorporated into cellulose nanocrystals modified with the cationic surfactant cetyltrimethylammonium bromide to bind a large amount of curcumin, and the amount of curcumin added ranges from 80% to 96% [51]. Moreover, curcumin, as an antioxidant, has great application in alleviating oxidative stress, such as diabetic nephropathy and AKI [52][53]. Therefore, cellulose nanocrystals with certain modifications have great potential and innovative applications in the treatment of kidney diseases.
4. Alginate
Brown algae are the largest group of marine macroalgae, and alginates are the most abundant natural marine polymers derived from brown algae [54]. Alginate is a non-branched polysaccharide consisting of (1,4) linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) in a non-repeating block. It consists of M residues and G residues that form M blocks or G blocks, respectively, or alternately, both form MG blocks (Figure 3) [55]. Alginate has a wide range of applications, from textile and food technology to biomedical and chemical engineering [56][57][58]. It has important applications in wound dressings; when applied to wounds, it forms a protective gel layer that promotes wound healing and tissue regeneration and maintains a stable temperature. In addition, alginate has applications in drug delivery due to its variable density and fiber composition, which make it easy to control the rate of drug release [59]. In addition, alginate can be easily manipulated through the simple addition of crosslinking agents, such as divalent calcium ions, for the development of different formulations of carriers [60]. Among the various alginate formulations, alginate microspheres have been extensively studied for their ability to encapsulate molecules with different properties. It is worth noting that alginate itself has no renal-targeting properties. However, due to the presence of open-function M and G groups, it can react with other cationic polymers, such as chitosan, thus achieving renal targeting ability and the encapsulation of drugs [61].
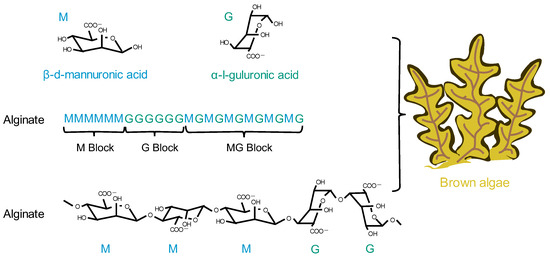
Figure 3. The chemical structural formula of alginate and its origin.
Based on the above, alginate nanoparticles have specific applications and great potential for renal drug delivery. T. I. M. Ragab et al. [62] used alginate-encapsulated carvacrol to form nanoemulsions in order to attenuate cisplatin-induced nephrotoxicity in rats. The prepared nanoemulsions were predominantly spherical, varying in particle size between 14 and 30 nm, and negatively charged. The nanoemulsions succeeded in significantly alleviating oxidative stress and inflammation in the kidneys of model rats, showing good renoprotective properties. In a study conducted by S. Heidarisasan et al. [63], the researchers utilized alginate and chitosan together to load insulin for the treatment of diabetic nephropathy in rats. The nanoparticles had a particle size of 533 nm, a zeta potential of 20 mv, and a loading capacity of 48.83%. After oral administration of the nanoparticles, this formulation successfully reduced the blood glucose and advanced glycosylation end-product levels in rats with diabetic nephropathy and improved oxidative stress conditions in the kidneys. Shanguo Zhang et al. [64] used a W/O microfluidic emulsion template method to prepare highly spherical calcium alginate microspheres in order to encapsulate a histone deacetylase inhibitor for AKI therapy. The shape of the nanoparticles they prepared was highly spherical. The in vivo results showed that the calcium alginate microspheres loaded with histone deacetylase inhibitors were effective in attenuating the inflammatory response and macrophage infiltration of the kidney.
References
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96.
- Xu, H.; Li, S.; Liu, Y.-S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target Ther. 2022, 7, 231.
- Chandra Hembram, K.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314.
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737.
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Jeevanandam, J.; Pan, S.; Rodrigues, J.; Elkodous, M.A.; Danquah, M.K. Medical applications of biopolymer nanofibers. Biomater. Sci. 2022, 10, 4107–4118.
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388.
- Liu, K.; Zhuang, Y.; Chen, J.; Yang, G.; Dai, L. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254.
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Li, H.; Dai, W.; Liu, Z.; He, L. Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals 2022, 15, 1494.
- Aghbashlo, M.; Amiri, H.; Moosavi Basri, S.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023, 41, 785–797.
- Ma, B.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Guo, Z. Preparation of imidazole acids grafted chitosan with enhanced antioxidant, antibacterial and antitumor activities. Carbohydr. Polym. 2023, 315, 120978.
- Zhang, Y.; Kang, R.; Zhang, X.; Pang, G.; Li, L.; Han, C.; Liu, B.; Xue, X.; Liu, J.; Sun, T.; et al. A programmable oral bacterial hydrogel for controllable production and release of nanovaccine for tumor immunotherapy. Biomaterials 2023, 299, 122147.
- Cheah, W.Y.; Show, P.-L.; Ng, I.S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577.
- Hosseini, M.; Amiri, M.; Ghanbari, M.; Mahdi, M.A.; Abdulsahib, W.K.; Salavati-Niasari, M. Drug delivery based on chitosan, β-cyclodextrin and sodium carboxymethyl cellulose as well as nanocarriers for advanced leukemia treatment. Biomed. Pharmacother. 2022, 153, 113369.
- Alhodieb, F.S.; Barkat, M.A.; Barkat, H.A.; Hadi, H.A.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469.
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286.
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108.
- Ahmad, M.; Taweel, G.M.A.; Hidayathulla, S. Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int. J. Biol. Macromol. 2018, 108, 591–597.
- Pang, M.; Duan, S.; Zhao, M.; Jiao, Q.; Bai, Y.; Yu, L.; Du, B.; Cheng, G. Co-delivery of celastrol and lutein with pH sensitive nano micelles for treating acute kidney injury. Toxicol. Appl. Pharmacol. 2022, 450, 116155.
- Liu, D.; Shu, G.; Jin, F.; Qi, J.; Xu, X.; Du, Y.; Yu, H.; Wang, J.; Sun, M.; You, Y.; et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, eabb7422.
- Robert, A.; Meunier, B. How to Define a Nanozyme. ACS Nano 2022, 16, 6956–6959.
- Liu, Z.; Xie, L.; Qiu, K.; Liao, X.; Rees, T.W.; Zhao, Z.; Ji, L.; Chao, H. An Ultrasmall RuO2 Nanozyme Exhibiting Multienzyme-like Activity for the Prevention of Acute Kidney Injury. ACS Appl. Mater. Interfaces 2020, 12, 31205–31216.
- Shen, P.; Tang, Q.; Chen, X.; Li, Z. Nanocrystalline cellulose extracted from bast fibers: Preparation, characterization, and application. Carbohydr. Polym. 2022, 290, 119462.
- Wang, Q.; Zhou, R.; Sun, J.; Liu, J.; Zhu, Q. Naturally Derived Janus Cellulose Nanomaterials: Anisotropic Cellulose Nanomaterial Building Blocks and Their Assembly into Asymmetric Structures. ACS Nano 2022, 16, 13468–13491.
- Keller, M.B.; Sørensen, T.H.; Krogh, K.B.R.M.; Wogulis, M.; Borch, K.; Westh, P. Activity of fungal β-glucosidases on cellulose. Biotechnol. Biofuels 2020, 13, 121.
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668.
- Rincón-Iglesias, M.; Lizundia, E.; Lanceros-Méndez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795.
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290.
- Ghilan, A.; Nicu, R.; Ciolacu, D.E.; Ciolacu, F. Insight into the Latest Medical Applications of Nanocellulose. Materials 2023, 16, 4447.
- Khan, A.; Jawaid, M.; Kian, L.K.; Khan, A.A.P.; Asiri, A.M. Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers 2021, 13, 1835.
- Baseer, R.A.; Dacrory, S.; El Gendy, M.A.M.; Ewies, E.F.; Kamel, S. A biodegradable film based on cellulose and thiazolidine bearing UV shielding property. Sci. Rep. 2022, 12, 7887.
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169.
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689.
- Chen, R.; Ma, Z.H.; Sun, D.Y.; Wang, X.; Han, Y. Cellulose I nanocrystals (CNCs I) prepared in mildly acidic lithium bromide trihydrate (MALBTH) and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2022, 201, 59–66.
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 989–1002.
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374.
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826.
- Wang, J.; Liu, Q.; Gong, J.; Wan, Z.; Zhou, J.; Chang, C.; Zhang, D. Micropatterned Hydrogels with Highly Ordered Cellulose Nanocrystals for Visually Monitoring Cardiomyocytes. Small 2022, 18, e2202235.
- Xu, J.; Zhang, J.; Zhang, F.; Zhang, L. Copolymer-Functionalized Cellulose Nanocrystals as a pH- and NIR-Triggered Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. Biomacromolecules 2022, 23, 4308–4317.
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072.
- Zhang, Q.; Wang, M.; Mu, G.; Ren, H.; He, C.; Xie, Q.; Liu, Q.; Wang, J.; Cha, R. Adsorptivity of cationic cellulose nanocrystals for phosphate and its application in hyperphosphatemia therapy. Carbohydr. Polym. 2021, 255, 117335.
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170.
- Gutekunst, L. An Update on Phosphate Binders: A Dietitian’s Perspective. J. Ren. Nutr. 2016, 26, 209–218.
- Wong, S.; Alidori, S.; Mello, B.P.; Almeida, B.A.; Ulmert, D.; Brendel, M.B.; Scheinberg, D.A.; McDevitt, M.R. Fibrillar pharmacology of functionalized nanocellulose. Sci. Rep. 2021, 11, 157.
- Zhao, X.; Wang, Q.; Zhu, G.; Ma, J.; Lin, N. Size effect of cellulose nanocrystals in cellular internalization and exosome-packaging exocytosis. Carbohydr. Polym. 2022, 298, 120131.
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676.
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269.
- Zhu, X.; Xu, X.; Du, C.; Su, Y.; Yin, L.; Tan, X.; Liu, H.; Wang, Y.; Xu, L.; Xu, X. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Biomed. Pharmacother. 2022, 153, 113438.
- Wei, H.; Jiang, D.; Yu, B.; Ni, D.; Li, M.; Long, Y.; Ellison, P.A.; Siamof, C.M.; Cheng, L.; Barnhart, T.E.; et al. Nanostructured polyvinylpyrrolidone-curcumin conjugates allowed for kidney-targeted treatment of cisplatin induced acute kidney injury. Bioact. Mater. 2023, 19, 282–291.
- Mrudulakumari Vasudevan, U.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158.
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305.
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and functional assessment of alginate fibers prepared by metal-calcium ion complex coagulation bath. Carbohydr. Polym. 2020, 232, 115693.
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369.
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436.
- He, Q.; Tong, T.; Yu, C.; Wang, Q. Advances in Algin and Alginate-Hybrid Materials for Drug Delivery and Tissue Engineering. Mar. Drugs 2022, 21, 14.
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627.
- Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride-In Vitro and In Vivo Evaluation. Molecules 2017, 22, 182.
- Ragab, T.I.M.; Zoheir, K.M.A.; Mohamed, N.A.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Abdelhameed, M.F.; Farrag, A.R.H.; Elshamy, A.I. Cytoprotective potentialities of carvacrol and its nanoemulsion against cisplatin-induced nephrotoxicity in rats: Development of nano-encasulation form. Heliyon 2022, 8, e09198.
- Heidarisasan, S.; Ziamajidi, N.; Karimi, J.; Abbasalipourkabir, R. Effects of insulin-loaded chitosan-alginate nanoparticles on RAGE expression and oxidative stress status in the kidney tissue of rats with type 1 diabetes. Iran. J. Basic. Med. Sci. 2018, 21, 1035–1042.
- Zhang, S.; Wang, X.; Man, J.; Li, J.; Cui, X.; Zhang, C.; Shi, W.; Li, D.; Zhang, S.; Li, J. Histone Deacetylase Inhibitor-loaded Calcium Alginate Microspheres for Acute Kidney Injury Treatment. ACS Appl. Bio. Mater. 2020, 3, 6457–6465.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
715
Revisions:
3 times
(View History)
Update Date:
31 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




