Kidney disease has become a serious public health problem throughout the world, and its treatment and management constitute a huge global economic burden. Currently, the main clinical treatments are not sufficient to cure kidney diseases. During its development, nanotechnology has shown unprecedented potential for application to kidney diseases. However, nanotechnology has disadvantages such as high cost and poor bioavailability. In contrast, biopolymers are not only widely available but also highly bioavailable. Therefore, biopolymer-based nanosystems offer new promising solutions for the treatment of kidney diseases. This paper reviews the biopolymer-based nanosystems that have been used for renal diseases and describes strategies for the specific, targeted delivery of drugs to the kidney as well as the physicochemical properties of the nanoparticles that affect the targeting success.
- nanoparticles
- biopolymer
- drug delivery systems
- kidney
- chitosan
- cellulose
- alginate
1. Introduction
2. Chitosan
Chitosan is a highly abundant natural biopolymer derived from the exoskeletons of crustaceans such as lobsters and crabs, insects, and fungal cell walls [80][10]. Chitosan is a polysaccharide formed through the deacetylation of chitin, and its chemical structure consists of N-acetylglucosamine and D-glucosamine monomers (Figure 4Figure 1). Chitosan’s molecular formula is C6H11NO4X2. Chitosan is formed after the N-deacetylation of chitin in reaction with a concentrated NaOH solution [81][11]. After deacetylation, as compared to chitin, chitosan acquires a higher water solubility and better chemical potential due to the free amino group [82][12]. The amino group on the surface of chitosan results in its positive charge [42][7]. This is of great interest for drug delivery to the kidney, as positively charged substances are more easily transported across the glomerular filtration barrier [63][13]. With continuous technological progress, the third generation of chitosan was introduced in the form of chitosan oligosaccharides, and chitosan became a designable bioactive compound, which greatly enhanced its application prospects [83][14]. At present, chitosan has a wide range of applications, including not only antibacterial, antitumor, and antioxidant applications [84–86][15][16][17] but also drug delivery, especially in its nanoform [87–89][18][19][20]. Chitosan nanoparticles are easy to produce, have a low toxicity, and are highly stable, biocompatible, and biodegradable. In addition, chitosan nanoparticles can release their encapsulated chemicals in a controlled manner [90][21]. As previously mentioned, chitosan with a low molecular weight can be selectively absorbed by megalin receptors in the kidney. Consequently, both chitosan nanoparticles and hybridized nanoparticles containing chitosan are effectively and selectively distributed in the kidney. All these advantages make chitosan nanoparticles an excellent vehicle for drug delivery to the kidney.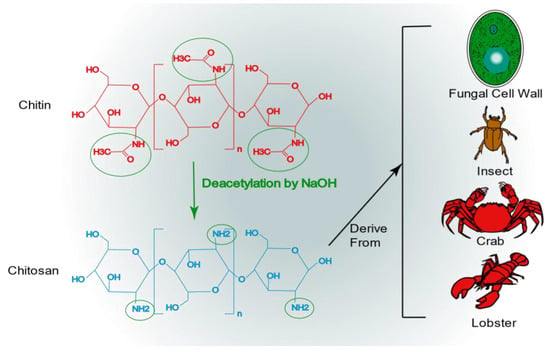

Figure 1. The chemical structure of chitosan and its origin.
Nanozymes are a focal topic of current research. Nanozymes are nanomaterials with an enzyme-like activity that have effective catalytic activity and a specific mechanism for a given reaction [96][25]. Z. Liu et al. [97][26] designed an ultra-small nanoenzyme for the treatment of AKI, and they successfully synthesized the nanoenzyme with an average particle size of 2 nm using chitosan, ruthenium(III) chloride trihydrate, and acetic acid with a solvothermal method. In addition, the nanoenzyme possesses multien-zyme-like activity (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)) and acts as an antioxidant to effectively scavenge ROS in kidney cells for the treatment of AKI. The excellent antioxidant properties, biostability, biocompatibility, and renal accumulation of this nanoenzyme make it promising for further applications in other kidney diseases caused by ROS, such as diabetic kidney disease.
3. Cellulose
Cellulose is by far the most abundant, most common, and renewable natural biopolymer, which is derived from wood, cotton, bacteria, and fungi [98,99][27][28]. Cellulose is a polysaccharide with a linear structure consisting of multiple D-glucose units linked by β-1,4 glycosidic bonds (Figure 5Figure 2) [100][29]. The cellulose molecular formula is (C6H10O5)n. Cellulose has a highly biocompatible and biodegradable nature, making it non-toxic [101][30]. Although cellulose is insoluble in water, which may limit its application in drug delivery, a series of cellulose derivatives can be formed through specific chemical reactions to improve its water solubility [102][31]. There are crystalline and amorphous regions in cellulose, and nanocrystalline cellulose is pure cellulose in a crystalline form with nanoscale dimensions [103][32]. The preparation methods of nanocrystalline cellulose mainly include acid hydrolysis, esterification using concentrated organic acids, (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl(TEMPO)-mediated oxidation, microbial or enzymatic hydrolysis, and periodate oxidation [104][33]. Nanocrystalline cellulose is a rod-shaped particle with an excellent aspect ratio, with a width of approximately 5–30 nm and a length of approximately 100–500 nm [105][34]. It also has superior axial Young’s modulus, high specific surface area, high transparency, and a number of other excellent properties, References [106,107][35][36]. As a result, nanocrystalline cellulose is widely used not only in drug delivery but also in many fields, such as papermaking, food production, and electronics [108–110][37][38][39]. In the kidney, nanocrystalline cellulose with a high aspect ratio (>10) is aligned with blood flow, filtered through the glomerulus, and subsequently reabsorbed by the renal unit from the tubular border. Due to the pharmacokinetic profile of the delivery platform, drugs bound to nanocrystalline cellulose are delivered to renal tubular cells [111][40]. Therefore, nanocrystalline cellulose has tremendous potential for renal drug delivery.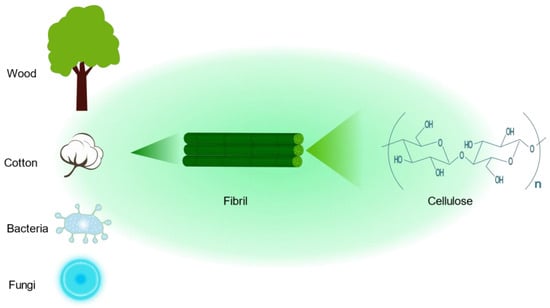
Figure 2. The chemical structure of cellulose and its sources.
There are a large number of extensive studies on the synthesis, characterization, and biological interactions of cellulose nanocrystals, as well as the successful loading of various hydrophobic drugs. However, there are few studies on the use of these drugs for the treatment of renal diseases [120,121][49][50]. For example, curcumin, a polyphenolic compound with antioxidant properties, can be incorporated into cellulose nanocrystals modified with the cationic surfactant cetyltrimethylammonium bromide to bind a large amount of curcumin, and the amount of curcumin added ranges from 80% to 96% [122][51]. Moreover, curcumin, as an antioxidant, has great application in alleviating oxidative stress, such as diabetic nephropathy and AKI [123,124][52][53]. Therefore, cellulose nanocrystals with certain modifications have great potential and innovative applications in the treatment of kidney diseases.
4. Alginate
Brown algae are the largest group of marine macroalgae, and alginates are the most abundant natural marine polymers derived from brown algae [125][54]. Alginate is a non-branched polysaccharide consisting of (1,4) linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) in a non-repeating block. It consists of M residues and G residues that form M blocks or G blocks, respectively, or alternately, both form MG blocks (Figure 6Figure 3) [126][55]. Alginate has a wide range of applications, from textile and food technology to biomedical and chemical engineering [127–129][56][57][58]. It has important applications in wound dressings; when applied to wounds, it forms a protective gel layer that promotes wound healing and tissue regeneration and maintains a stable temperature. In addition, alginate has applications in drug delivery due to its variable density and fiber composition, which make it easy to control the rate of drug release [130][59]. In addition, alginate can be easily manipulated through the simple addition of crosslinking agents, such as divalent calcium ions, for the development of different formulations of carriers [131][60]. Among the various alginate formulations, alginate microspheres have been extensively studied for their ability to encapsulate molecules with different properties. It is worth noting that alginate itself has no renal-targeting properties. However, due to the presence of open-function M and G groups, it can react with other cationic polymers, such as chitosan, thus achieving renal targeting ability and the encapsulation of drugs [132][61].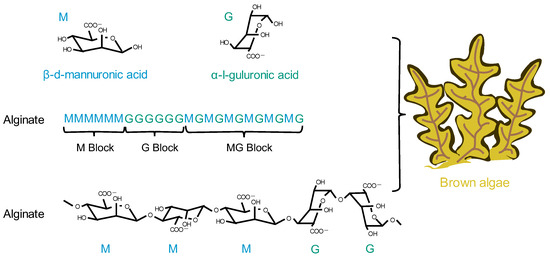
References
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96.
- Xu, H.; Li, S.; Liu, Y.-S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target Ther. 2022, 7, 231.
- Chandra Hembram, K.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314.
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737.
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Jeevanandam, J.; Pan, S.; Rodrigues, J.; Elkodous, M.A.; Danquah, M.K. Medical applications of biopolymer nanofibers. Biomater. Sci. 2022, 10, 4107–4118.
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388.
- Liu, K.; Zhuang, Y.; Chen, J.; Yang, G.; Dai, L. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254.
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Li, H.; Dai, W.; Liu, Z.; He, L. Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals 2022, 15, 1494.
- Aghbashlo, M.; Amiri, H.; Moosavi Basri, S.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023, 41, 785–797.
- Ma, B.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Guo, Z. Preparation of imidazole acids grafted chitosan with enhanced antioxidant, antibacterial and antitumor activities. Carbohydr. Polym. 2023, 315, 120978.
- Zhang, Y.; Kang, R.; Zhang, X.; Pang, G.; Li, L.; Han, C.; Liu, B.; Xue, X.; Liu, J.; Sun, T.; et al. A programmable oral bacterial hydrogel for controllable production and release of nanovaccine for tumor immunotherapy. Biomaterials 2023, 299, 122147.
- Cheah, W.Y.; Show, P.-L.; Ng, I.S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577.
- Hosseini, M.; Amiri, M.; Ghanbari, M.; Mahdi, M.A.; Abdulsahib, W.K.; Salavati-Niasari, M. Drug delivery based on chitosan, β-cyclodextrin and sodium carboxymethyl cellulose as well as nanocarriers for advanced leukemia treatment. Biomed. Pharmacother. 2022, 153, 113369.
- Alhodieb, F.S.; Barkat, M.A.; Barkat, H.A.; Hadi, H.A.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469.
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286.
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108.
- Ahmad, M.; Taweel, G.M.A.; Hidayathulla, S. Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int. J. Biol. Macromol. 2018, 108, 591–597.
- Pang, M.; Duan, S.; Zhao, M.; Jiao, Q.; Bai, Y.; Yu, L.; Du, B.; Cheng, G. Co-delivery of celastrol and lutein with pH sensitive nano micelles for treating acute kidney injury. Toxicol. Appl. Pharmacol. 2022, 450, 116155.
- Liu, D.; Shu, G.; Jin, F.; Qi, J.; Xu, X.; Du, Y.; Yu, H.; Wang, J.; Sun, M.; You, Y.; et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, eabb7422.
- Robert, A.; Meunier, B. How to Define a Nanozyme. ACS Nano 2022, 16, 6956–6959.
- Liu, Z.; Xie, L.; Qiu, K.; Liao, X.; Rees, T.W.; Zhao, Z.; Ji, L.; Chao, H. An Ultrasmall RuO2 Nanozyme Exhibiting Multienzyme-like Activity for the Prevention of Acute Kidney Injury. ACS Appl. Mater. Interfaces 2020, 12, 31205–31216.
- Shen, P.; Tang, Q.; Chen, X.; Li, Z. Nanocrystalline cellulose extracted from bast fibers: Preparation, characterization, and application. Carbohydr. Polym. 2022, 290, 119462.
- Wang, Q.; Zhou, R.; Sun, J.; Liu, J.; Zhu, Q. Naturally Derived Janus Cellulose Nanomaterials: Anisotropic Cellulose Nanomaterial Building Blocks and Their Assembly into Asymmetric Structures. ACS Nano 2022, 16, 13468–13491.
- Keller, M.B.; Sørensen, T.H.; Krogh, K.B.R.M.; Wogulis, M.; Borch, K.; Westh, P. Activity of fungal β-glucosidases on cellulose. Biotechnol. Biofuels 2020, 13, 121.
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668.
- Rincón-Iglesias, M.; Lizundia, E.; Lanceros-Méndez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795.
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290.
- Ghilan, A.; Nicu, R.; Ciolacu, D.E.; Ciolacu, F. Insight into the Latest Medical Applications of Nanocellulose. Materials 2023, 16, 4447.
- Khan, A.; Jawaid, M.; Kian, L.K.; Khan, A.A.P.; Asiri, A.M. Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers 2021, 13, 1835.
- Baseer, R.A.; Dacrory, S.; El Gendy, M.A.M.; Ewies, E.F.; Kamel, S. A biodegradable film based on cellulose and thiazolidine bearing UV shielding property. Sci. Rep. 2022, 12, 7887.
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169.
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689.
- Chen, R.; Ma, Z.H.; Sun, D.Y.; Wang, X.; Han, Y. Cellulose I nanocrystals (CNCs I) prepared in mildly acidic lithium bromide trihydrate (MALBTH) and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2022, 201, 59–66.
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 989–1002.
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374.
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826.
- Wang, J.; Liu, Q.; Gong, J.; Wan, Z.; Zhou, J.; Chang, C.; Zhang, D. Micropatterned Hydrogels with Highly Ordered Cellulose Nanocrystals for Visually Monitoring Cardiomyocytes. Small 2022, 18, e2202235.
- Xu, J.; Zhang, J.; Zhang, F.; Zhang, L. Copolymer-Functionalized Cellulose Nanocrystals as a pH- and NIR-Triggered Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. Biomacromolecules 2022, 23, 4308–4317.
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072.
- Zhang, Q.; Wang, M.; Mu, G.; Ren, H.; He, C.; Xie, Q.; Liu, Q.; Wang, J.; Cha, R. Adsorptivity of cationic cellulose nanocrystals for phosphate and its application in hyperphosphatemia therapy. Carbohydr. Polym. 2021, 255, 117335.
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170.
- Gutekunst, L. An Update on Phosphate Binders: A Dietitian’s Perspective. J. Ren. Nutr. 2016, 26, 209–218.
- Wong, S.; Alidori, S.; Mello, B.P.; Almeida, B.A.; Ulmert, D.; Brendel, M.B.; Scheinberg, D.A.; McDevitt, M.R. Fibrillar pharmacology of functionalized nanocellulose. Sci. Rep. 2021, 11, 157.
- Zhao, X.; Wang, Q.; Zhu, G.; Ma, J.; Lin, N. Size effect of cellulose nanocrystals in cellular internalization and exosome-packaging exocytosis. Carbohydr. Polym. 2022, 298, 120131.
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676.
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269.
- Zhu, X.; Xu, X.; Du, C.; Su, Y.; Yin, L.; Tan, X.; Liu, H.; Wang, Y.; Xu, L.; Xu, X. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Biomed. Pharmacother. 2022, 153, 113438.
- Wei, H.; Jiang, D.; Yu, B.; Ni, D.; Li, M.; Long, Y.; Ellison, P.A.; Siamof, C.M.; Cheng, L.; Barnhart, T.E.; et al. Nanostructured polyvinylpyrrolidone-curcumin conjugates allowed for kidney-targeted treatment of cisplatin induced acute kidney injury. Bioact. Mater. 2023, 19, 282–291.
- Mrudulakumari Vasudevan, U.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158.
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305.
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and functional assessment of alginate fibers prepared by metal-calcium ion complex coagulation bath. Carbohydr. Polym. 2020, 232, 115693.
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369.
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436.
- He, Q.; Tong, T.; Yu, C.; Wang, Q. Advances in Algin and Alginate-Hybrid Materials for Drug Delivery and Tissue Engineering. Mar. Drugs 2022, 21, 14.
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627.
- Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride-In Vitro and In Vivo Evaluation. Molecules 2017, 22, 182.
- Ragab, T.I.M.; Zoheir, K.M.A.; Mohamed, N.A.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Abdelhameed, M.F.; Farrag, A.R.H.; Elshamy, A.I. Cytoprotective potentialities of carvacrol and its nanoemulsion against cisplatin-induced nephrotoxicity in rats: Development of nano-encasulation form. Heliyon 2022, 8, e09198.
- Heidarisasan, S.; Ziamajidi, N.; Karimi, J.; Abbasalipourkabir, R. Effects of insulin-loaded chitosan-alginate nanoparticles on RAGE expression and oxidative stress status in the kidney tissue of rats with type 1 diabetes. Iran. J. Basic. Med. Sci. 2018, 21, 1035–1042.
- Zhang, S.; Wang, X.; Man, J.; Li, J.; Cui, X.; Zhang, C.; Shi, W.; Li, D.; Zhang, S.; Li, J. Histone Deacetylase Inhibitor-loaded Calcium Alginate Microspheres for Acute Kidney Injury Treatment. ACS Appl. Bio. Mater. 2020, 3, 6457–6465.
