
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | François DUPUIS | -- | 5310 | 2023-08-24 14:36:33 | | | |
| 2 | Lindsay Dong | Meta information modification | 5310 | 2023-08-25 03:19:44 | | |
Video Upload Options
The AT1 receptor has mainly been associated with the pathological effects of the renin-angiotensin system (RAS) (e.g., hypertension, heart and kidney diseases), and constitutes a major therapeutic target. In contrast, the AT2 receptor is presented as the protective arm of this RAS, and its targeting via specific agonists is mainly used to counteract the effects of the AT1 receptor. The discovery of a local RAS has highlighted the importance of the balance between AT1/AT2 receptors at the tissue level. Disruption of this balance is suggested to be detrimental. The fine tuning of this balance is not limited to the regulation of the level of expression of these two receptors. Other mechanisms still largely unexplored, such as S-nitrosation of the AT1 receptor, homo- and heterodimerization, and the use of AT1 receptor-biased agonists, may significantly contribute to and/or interfere with the settings of this AT1/AT2 equilibrium.
1. Introduction
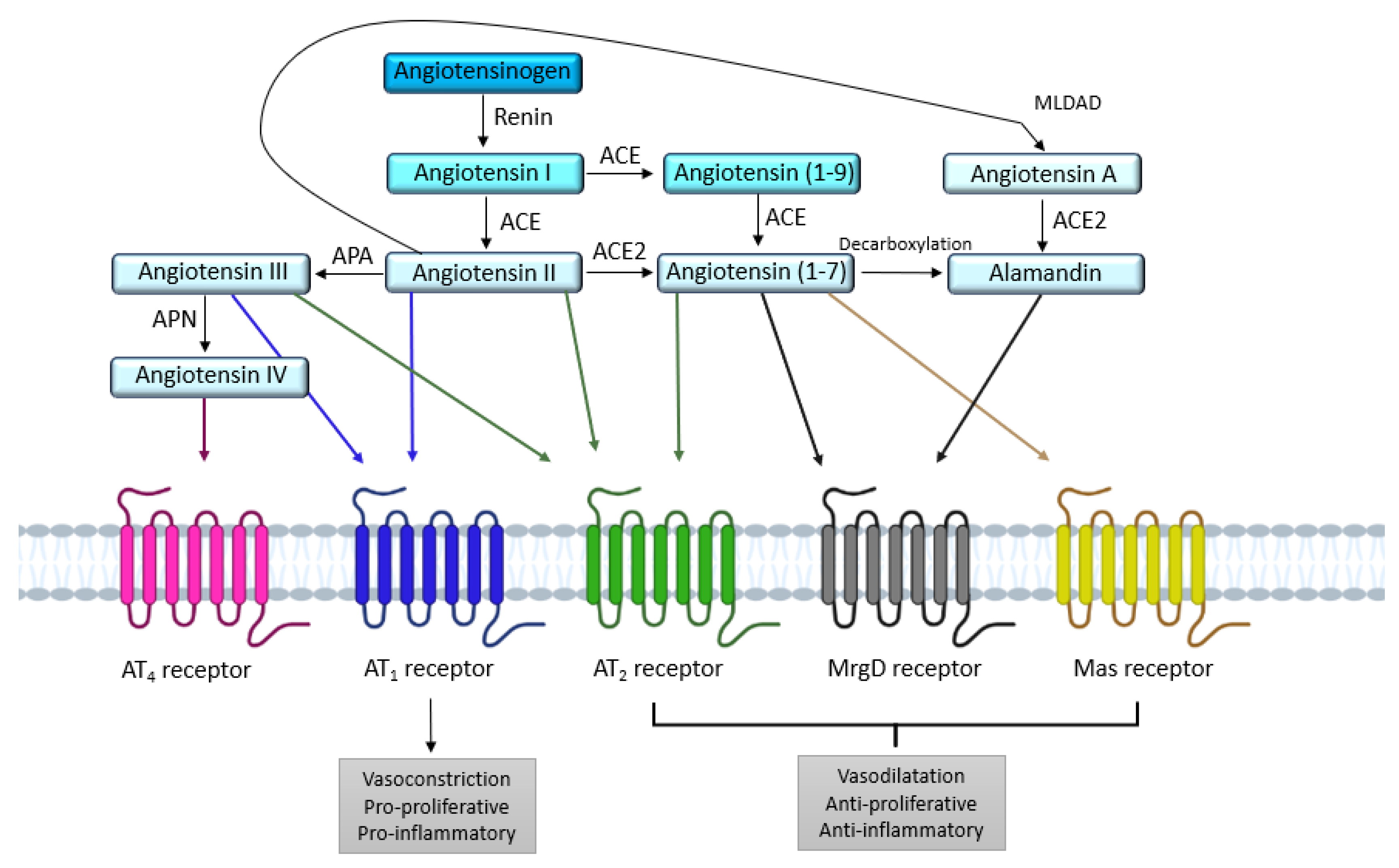
2. AT1 and AT2 Angiotensin II Receptors
2.1. AT1 Receptor
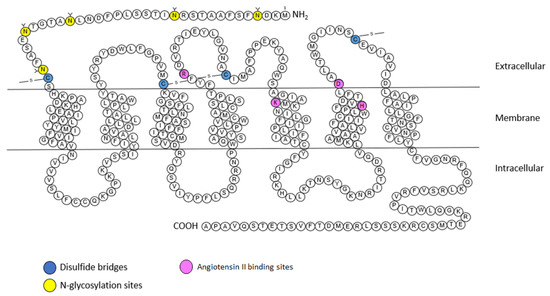
2.1.1. Structure
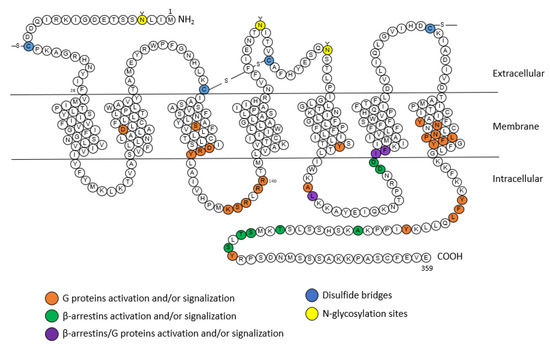
2.1.2. Signaling
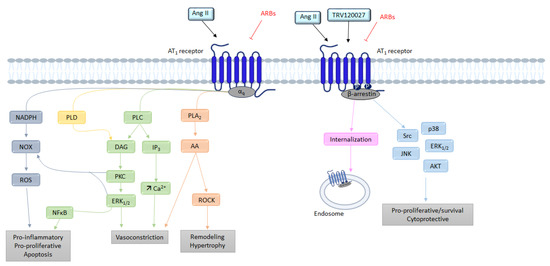
G Protein Pathway
β-Arrestins
NADPH
2.2. AT2 Receptor
2.2.1. Structure
2.2.2. Signaling
G Protein Pathway
Bradykinin
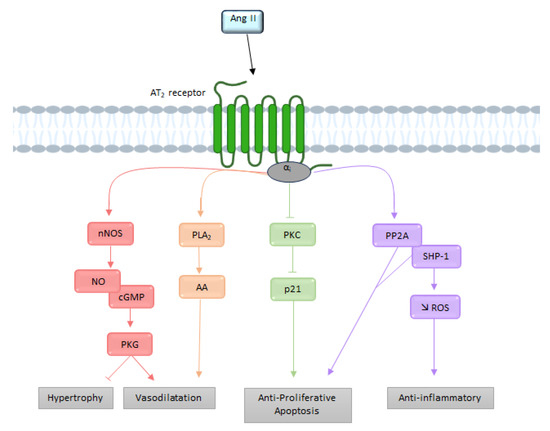
3. The Functional AT1/AT2 Receptors Balance
3.1. Systemic Cardiovascular Impact
3.2. AT1/AT2 Balance in the Brain and Cerebral Circulation
3.2.1. Cerebral Circulation
3.2.2. Cardiovascular Regulation
3.2.3. Neuroinflammation
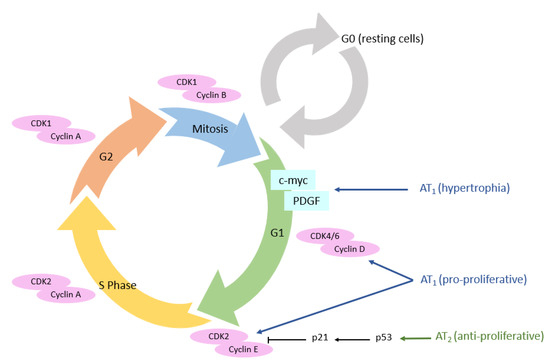
3.3. Cellular Cycle
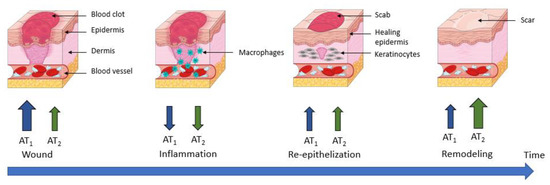
3.4. Wound Healing
4. Mechanisms Regulating the AT1/AT2 Functional Balance
4.1. Functional Opposition vs. Expression Level
4.2. Direct AT1/AT2 Receptors Interactions
4.2.1. AT1 Receptor Dimerization
4.2.2. AT2 Receptor Dimerization
4.3. Post-Translational Modifications
4.3.1. N-Glycosylation
4.3.2. Phosphorylation
4.3.3. S-Nitrosation
5. Possible Ways to Tune the AT1/AT2 Functional Balance
5.1. Pushing the Balance Using Agonist/Antagonist Ligands
5.2. Selective Activation of the β-Arrestin Pathway
6. Conclusions
References
- Henrion, D.; Chillon, J.-M.; Capdeville-Atkinson, C.; Vinceneux-Feugier, M.; Atkinson, J. Chronic treatment with the angiotensin I converting enzyme inhibitor, perindopril, protects in vitro carbachol-induced vasorelaxation in a rat model of vascular calcium overload. Br. J. Pharmacol. 1991, 104, 966–972.
- Lartaud, I.; Bray-des-Boscs, L.; Chillon, J.M.; Atkinson, J.; Capdeville-Atkinson, C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am. J. Physiol. Heart Circ. Physiol. 1993, 264, H851–H858.
- Régrigny, O.; Atkinson, J.; Capdeville-Atkinson, C.; Limiñana, P.; Chillon, J.-M. Effect of Lovastatin on Cerebral Circulation in Spontaneously Hypertensive Rats. Hypertension 2000, 35, 1105–1110.
- Atkinson, J. Stroke, high blood pressure and the renin–angiotensin–aldosterone system—New developments. Front. Pharm. 2011, 2, 22.
- Tigerstedt, R.; Bergman, P.Q. Niere und Kreislauf 1. Skand. Arch. Für Physiol. 1898, 8, 223–271.
- Guyton, A.C. Blood Pressure Control—Special Role of the Kidneys and Body Fluids. Science 1991, 252, 1813–1816.
- Skeggs, L.T.; Lentz, K.E.; Gould, A.B.; Hochstrasser, H.; Kahn, J.R. Biochemistry and kinetics of the renin-angiotensin system. Fed. Proc. 1967, 26, 42–47.
- Murphy, T.J.; Alexander, R.W.; Griendling, K.K.; Runge, M.S.; Bernstein, K.E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 1991, 351, 233–236.
- Mukoyama, M.; Nakajima, M.; Horiuchi, M.; Sasamura, H.; Pratt, R.E.; Dzau, V.J. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J. Biol. Chem. 1993, 268, 24539–24542.
- Sevá Pessôa, B.; van der Lubbe, N.; Verdonk, K.; Roks, A.J.M.; Hoorn, E.J.; Danser, A.H.J. Key developments in renin–angiotensin–aldosterone system inhibition. Nat. Rev. Nephrol. 2013, 9, 26–36.
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent Progress in Angiotensin II Type 2 Receptor Research in the Cardiovascular System. Hypertension 1999, 33, 613–621.
- Gasparo, M.D.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International Union of Pharmacology. XXIII. The Angiotensin II Receptors. Pharmacol. Rev. 2000, 52, 415–472.
- Campbell, D.J.; Habener, J.F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J. Clin. Investig. 1986, 78, 31–39.
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 Receptor Signaling Pathways Mediating Physiological and Pathogenic Actions of Angiotensin II. Mol. Endocrinol. 2006, 20, 953–970.
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738.
- Pueyo, M.E.; N’Diaye, N.; Michel, J.-B. Angiotensin Il-elicited signal transduction via AT1 receptors in endothelial cells. Br. J. Pharmacol. 1996, 118, 79–84.
- Busche, S.; Gallinat, S.; Bohle, R.-M.; Reinecke, A.; Seebeck, J.; Franke, F.; Fink, L.; Zhu, M.; Sumners, C.; Unger, T. Expression of Angiotensin AT1 and AT2 Receptors in Adult Rat Cardiomyocytes after Myocardial Infarction. Am. J. Pathol. 2000, 157, 605–611.
- Siragy, H.M. AT1 and AT2 receptor in the kidney: Role in health and disease. Semin. Nephrol. 2004, 24, 93–100.
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortès, C. Expression of Angiotensin Type-1 (AT1) and Type-2 (AT2) Receptor mRNAs in the Adult Rat Brain: A Functional Neuroanatomical Review. Front. Neuroendocrinol. 1997, 18, 383–439.
- Johren, O.; Saavedra, J.M. Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wk-old rats. Am. J. Physiol. Endocrinol. Metab. 1996, 271, E104–E112.
- Zhang, H.; Unal, H.; Gati, C.; Han, G.W.; Liu, W.; Zatsepin, N.A.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; et al. Structure of the Angiotensin Receptor Revealed by Serial Femtosecond Crystallography. Cell 2015, 161, 833–844.
- Wingler, L.M.; Skiba, M.A.; McMahon, C.; Staus, D.P.; Kleinhenz, A.L.W.; Suomivuori, C.-M.; Latorraca, N.R.; Dror, R.O.; Lefkowitz, R.J.; Kruse, A.C. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 2020, 367, 888–892.
- Suomivuori, C.-M.; Latorraca, N.R.; Wingler, L.M.; Eismann, S.; King, M.C.; Kleinhenz, A.L.W.; Skiba, M.A.; Staus, D.P.; Kruse, A.C.; Lefkowitz, R.J.; et al. Molecular mechanism of biased signaling in a prototypical G protein–coupled receptor. Science. 2020, 367, 881–887.
- Aplin, M.; Bonde, M.M.; Hansen, J.L. Molecular determinants of angiotensin II type 1 receptor functional selectivity. J. Mol. Cell. Cardiol. 2009, 46, 15–24.
- Balakumar, P.; Jagadeesh, G. Structural determinants for binding, activation, and functional selectivity of the angiotensin AT1 receptor. J. Mol. Endocrinol. 2014, 53, R71–R92.
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 Receptors: Regulation, Signalling and Function. Blood Press. 2003, 12, 70–88.
- Rao, G.N.; Lassegue, B.; Alexander, R.W.; Griendling, K.K. Angiotensin 11 stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem. J. 1994, 299, 197–201.
- Touyz, R.M.; Schiffrin, E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000, 52, 639–672.
- Ohtsu, H.; Suzuki, H.; Nakashima, H.; Dhobale, S.; Frank, G.D.; Motley, E.D.; Eguchi, S. Angiotensin II Signal Transduction Through Small GTP-Binding Proteins: Mechanism and Significance in Vascular Smooth Muscle Cells. Hypertension 2006, 48, 534–540.
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13.
- Violin, J.D.; Lefkowitz, R.J. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007, 28, 416–422.
- Luttrell, L.M.; Lefkowitz, R.J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002, 115, 455–465.
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148.
- Touyz, R.M.; Briones, A.M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res. 2011, 34, 5–14.
- Tsutsumi, K.; Saavedra, J.M. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am. J. Physiol. 1991, 261, R209–R216.
- Ichiki, T.; Herold, C.L.; Kambayashi, Y.; Bardhan, S.; Inagami, T. Cloning of the cDNA and the genomic DNA of the mouse angiotensin II type 2 receptor. Biochim. Biophys. Acta (BBA) Biomembr. 1994, 1189, 247–250.
- Rompe, F.; Artuc, M.; Hallberg, A.; Alterman, M.; Ströder, K.; Thöne-Reineke, C.; Reichenbach, A.; Schacherl, J.; Dahlöf, B.; Bader, M.; et al. Direct Angiotensin II Type 2 Receptor Stimulation Acts Anti-Inflammatory Through Epoxyeicosatrienoic Acid and Inhibition of Nuclear Factor κB. Hypertension 2010, 55, 924–931.
- Zhang, H.; Han, G.W.; Batyuk, A.; Ishchenko, A.; White, K.L.; Patel, N.; Sadybekov, A.; Zamlynny, B.; Rudd, M.T.; Hollenstein, K.; et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature 2017, 544, 327–332.
- Feng, Y.-H.; Saad, Y.; Karnik, S.S. Reversible inactivation of AT2 angiotensin II receptor from cysteine-disulfide bond exchange. FEBS Lett. 2000, 484, 133–138.
- Heerding, J.N.; Yee, D.K.; Jacobs, S.L.; Fluharty, S.J. Mutational analysis of the angiotensin II type 2 receptor: Contribution of conserved extracellular amino acids. Regul. Pept. 1997, 72, 97–103.
- Yee, D.K.; Kisley, L.R.; Heerding, J.N.; Fluharty, S.J. Mutation of a conserved fifth transmembrane domain lysine residue (Lys215) attenuates ligand binding in the angiotensin II type 2 receptor. Brain Res. Mol. Brain Res. 1997, 51, 238–241.
- Turner, C.A.; Cooper, S.; Pulakat, L. Role of the His273 located in the sixth transmembrane domain of the Angiotensin II receptor subtype AT2 in ligand–receptor interaction. Biochem. Biophys. Res. Commun. 1999, 257, 704–707.
- Steckelings, U.M.; Kaschina, E.; Unger, T. The AT2 receptor—A matter of love and hate. Peptides 2005, 26, 1401–1409.
- Turu, G.; Szidonya, L.; Gáborik, Z.; Buday, L.; Spät, A.; Clark, A.J.L.; Hunyady, L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 2006, 580, 41–45.
- Padia, S.H.; Carey, R.M. AT2 receptors: Beneficial counter-regulatory role in cardiovascular and renal function. Pflug. Arch. Eur. J. Physiol. 2013, 465, 99–110.
- Bottari, S.P.; Taylor, V.; King, I.N.; Bogdal, Y.; Whitebread, S.; de Gasparo, M. Angiotensin II AT2 receptors do not interact with guanine nucleotide binding proteins. Eur. J. Pharmacol. Mol. Pharmacol. 1991, 207, 157–163.
- Zhang, J.; Pratt, R.E. The AT2 Receptor Selectively Associates with Giα2 and Giα3 in the Rat Fetus. J. Biol. Chem. 1996, 271, 15026–15033.
- Stennett, A.K.; Qiao, X.; Falone, A.E.; Koledova, V.V.; Khalil, R.A. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H745–H755.
- Vinturache, A.E.; Smith, F.G. Angiotensin type 1 and type 2 receptors during ontogeny: Cardiovascular and renal effects. Vasc. Pharmacol. 2014, 63, 145–154.
- Alexander, L.D.; Ding, Y.; Alagarsamy, S.; Cui, X. Angiotensin II stimulates fibronectin protein synthesis via a Gβγ/arachidonic acid-dependent pathway. Am. J. Physiol. Ren. Physiol. 2014, 307, F287–F302.
- Siragy, H.M.; Carey, R.M. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J. Clin. Investig. 1997, 100, 264–269.
- Tsutsumi, Y.; Matsubara, H.; Masaki, H.; Kurihara, H.; Murasawa, S.; Takai, S.; Miyazaki, M.; Nozawa, Y.; Ozono, R.; Nakagawa, K.; et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Investig. 1999, 104, 925–935.
- Yayama, K.; Hiyoshi, H.; Imazu, D.; Okamoto, H. Angiotensin II Stimulates Endothelial NO Synthase Phosphorylation in Thoracic Aorta of Mice With Abdominal Aortic Banding Via Type 2 Receptor. Hypertension 2006, 48, 958–964.
- Anderson, W.P.; Selig, S.E.; Korner, P.I. Role of Angiotensin II in the Hypertension Induced by Renal Artery Stenosis. Clin. Exp. Hypertens. Part A Theory Pract. 1984, 6, 299–314.
- Fischer-Ferraro, C.; Nahmod, V.E.; Goldstein, D.J.; Finkielman, S. Angiotensin and renin in rat and dog brain. J. Exp. Med. 1971, 133, 353–361.
- Barnes, J.M.; Steward, L.J.; Barber, P.C.; Barnes, N.M. Identification and characterisation of angiotensin II receptor subtypes in human brain. Eur. J. Pharmacol. 1993, 230, 251–258.
- Vincent, J.-M.; Kwan, Y.W.; Lung Chan, S.; Perrin-Sarrado, C.; Atkinson, J.; Chillon, J.-M. Constrictor and Dilator Effects of Angiotensin II on Cerebral Arterioles. Stroke 2005, 36, 2691–2695.
- Ohyama, K.; Yamano, Y.; Sano, T.; Nakagomi, Y.; Hamakubo, T.; Morishima, I.; Inagami, T. Disulfide bridges in extracellular domains of angiotensin II receptor type IA. Regul. Pept. 1995, 57, 141–147.
- Chen, D.; Jancovski, N.; Bassi, J.K.; Nguyen-Huu, T.-P.; Choong, Y.-T.; Palma-Rigo, K.; Davern, P.J.; Gurley, S.B.; Thomas, W.G.; Head, G.A.; et al. Angiotensin Type 1A Receptors in C1 Neurons of the Rostral Ventrolateral Medulla Modulate the Pressor Response to Aversive Stress. J. Neurosci. 2012, 32, 2051–2061.
- De Kloet, A.D.; Wang, L.; Ludin, J.A.; Smith, J.A.; Pioquinto, D.J.; Hiller, H.; Steckelings, U.M.; Scheuer, D.A.; Sumners, C.; Krause, E.G. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct. Funct. 2016, 221, 891–912.
- Gonzalez, A.D.; Wang, G.; Waters, E.M.; Gonzales, K.L.; Speth, R.C.; Van Kempen, T.A.; Marques-Lopes, J.; Young, C.N.; Butler, S.D.; Davisson, R.L.; et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 2012, 226, 489–509.
- Elsaafien, K.; de Kloet, A.D.; Krause, E.G.; Sumners, C. Brain Angiotensin Type-1 and Type-2 Receptors in Physiological and Hypertensive Conditions: Focus on Neuroinflammation. Curr. Hypertens. Rep. 2020, 22, 48.
- De Kloet, A.D.; Wang, L.; Pitra, S.; Hiller, H.; Smith, J.A.; Tan, Y.; Nguyen, D.; Cahill, K.M.; Sumners, C.; Stern, J.E.; et al. A Unique “Angiotensin-Sensitive” Neuronal Population Coordinates Neuroendocrine, Cardiovascular, and Behavioral Responses to Stress. J. Neurosci. 2017, 37, 3478–3490.
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. 2015, 128, 95–109.
- Kim, J.-H.; Afridi, R.; Cho, E.; Yoon, J.H.; Lim, Y.-H.; Lee, H.-W.; Ryu, H.; Suk, K. Soluble ANPEP Released From Human Astrocytes as a Positive Regulator of Microglial Activation and Neuroinflammation: Brain Renin–Angiotensin System in Astrocyte–Microglia Crosstalk. Mol. Cell. Proteom. 2022, 21, 100424.
- Rodriguez-Perez, A.I.; Borrajo, A.; Rodriguez-Pallares, J.; Guerra, M.J.; Labandeira-Garcia, J.L. Interaction between NADPH-oxidase and Rho-kinase in angiotensin II-induced microglial activation: NADPH-Oxidase and Rho-Kinase Interaction. Glia 2015, 63, 466–482.
- Campbell, G.J.; Hands, E.L.; Van de Pette, M. The Role of CDKs and CDKIs in Murine Development. Int. J. Mol. Sci. 2020, 21, 5343.
- Han, H.J.; Han, J.Y.; Heo, J.S.; Lee, S.H.; Lee, M.Y.; Kim, Y.H. ANG II-stimulated DNA synthesis is mediated by ANG II receptor-dependent Ca2+/PKC as well as EGF receptor-dependent PI3K/Akt/mTOR/p70S6K1 signal pathways in mouse embryonic stem cells. J. Cell. Physiol. 2007, 211, 618–629.
- Yu, L.; Meng, W.; Ding, J.; Cheng, M. Klotho inhibits angiotensin II-induced cardiomyocyte hypertrophy through suppression of the AT1R/beta catenin pathway. Biochem. Biophys. Res. Commun. 2016, 473, 455–461.
- Zhou, L.; Liu, Y. Wnt/β-catenin signaling and renin–angiotensin system in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 100–106.
- Fujihara, S.; Morishita, A.; Ogawa, K.; Tadokoro, T.; Chiyo, T.; Kato, K.; Kobara, H.; Mori, H.; Iwama, H.; Masaki, T. The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKα/mTOR pathway in vitro and in vivo. Oncotarget 2017, 8, 8536–8549.
- Samukawa, E.; Fujihara, S.; Oura, K.; Iwama, H.; Yamana, Y.; Tadokoro, T.; Chiyo, T.; Kobayashi, K.; Morishita, A.; Nakahara, M.; et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int. J. Oncol. 2017, 51, 1674–1684.
- Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Przylipiak, A.; Chabielska, E. The expression of the renin-angiotensin-aldosterone system in the skin and its effects on skin physiology and pathophysiology. J. Physiol. Pharmacol. 2019, 70.
- Silva, I.M.S.; Assersen, K.B.; Willadsen, N.N.; Jepsen, J.; Artuc, M.; Steckelings, U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp. Dermatol. 2020, 29, 891–901.
- Viswanathan, M.; Saavedra, J.M. Expression of angiotensin II AT2 receptors in the rat skin during experimental wound healing. Peptides 1992, 13, 783–786.
- Steckelings, U.M.; Henz, B.M.; Wiehstutz, S.; Unger, T.; Artuc, M. Differential expression of angiotensin receptors in human cutaneous wound healing. Br. J. Dermatol. 2005, 153, 887–893.
- Takeda, H.; Katagata, Y.; Kondo, S. Immunohistochemical study of angiotensin receptors in human anagen hair follicles and basal cell carcinoma. Br. J. Dermatol. 2002, 147, 276–280.
- Jiang, X.; Wu, F.; Xu, Y.; Yan, J.-X.; Wu, Y.-D.; Li, S.-H.; Liao, X.; Liang, J.-X.; Li, Z.-H.; Liu, H.-W. A novel role of angiotensin II in epidermal cell lineage determination: Angiotensin II promotes the differentiation of mesenchymal stem cells into keratinocytes through the p38 MAPK, JNK and JAK2 signalling pathways. Exp. Dermatol. 2019, 28, 59–65.
- Jadhav, S.S.; Sharma, N.; Meeks, C.J.; Mordwinkin, N.M.; Espinoza, T.B.; Roda, N.R.; DiZerega, G.S.; Hill, C.K.; Louie, S.G.; Rodgers, K.E. Effects of combined radiation and burn injury on the renin-angiotensin system: CRBI and renin-angiotensin system. Wound Repair. Regen. 2013, 21, 131–140.
- Kamiñska, M.; Mogielnicki, A.; Stankiewicz, A.; Kramkowski, K.; Domaniewski, T.; Buczko, W.; Chabielska, E. Angiotensin Ii Via At1 Receptor Accelerates Arterial Thrombosis In Renovascular Hypertensive Rats. J. Physiol. Pharmacol. 2005, 56, 571–585.
- Bernasconi, R.; Nyström, A. Balance and circumstance: The renin angiotensin system in wound healing and fibrosis. Cell. Signal. 2018, 51, 34–46.
- Yahata, Y.; Shirakata, Y.; Tokumaru, S.; Yang, L.; Dai, X.; Tohyama, M.; Tsuda, T.; Sayama, K.; Iwai, M.; Horiuchi, M.; et al. A Novel Function of Angiotensin II in Skin Wound Healing. J. Biol. Chem. 2006, 281, 13209–13216.
- Faghih, M.; Hosseini, S.M.; Smith, B.; Ansari, A.M.; Lay, F.; Ahmed, A.K.; Inagami, T.; Marti, G.P.; Harmon, J.W.; Walston, J.D.; et al. Knockout of Angiotensin AT2 receptors accelerates healing but impairs quality. Aging 2015, 7, 1185–1197.
- Karppinen, S.-M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Res 2019, 8, 787.
- Murphy, A.M.; Wong, A.L.; Bezuhly, M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenes. Tissue Repair. 2015, 8, 7.
- Ito, M.; Oliverio, M.I.; Mannon, P.J.; Best, C.F.; Maeda, N.; Smithies, O.; Coffman, T.M. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc. Natl. Acad. Sci. USA 1995, 92, 3521–3525.
- Daviet, L.; Lehtonen, J.Y.; Tamura, K.; Griese, D.P.; Horiuchi, M.; Dzau, V.J. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J. Biol. Chem. 1999, 274, 17058–17062.
- Lopez-Ilasaca, M.; Liu, X.; Tamura, K.; Dzau, V.J. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol. Biol. Cell. 2003, 14, 5038–5050.
- Mogi, M.; Iwai, M.; Horiuchi, M. Emerging Concepts of Regulation of Angiotensin II Receptors: New Players and Targets for Traditional Receptors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2532–2539.
- Wruck, C.J.; Funke-Kaiser, H.; Pufe, T.; Kusserow, H.; Menk, M.; Schefe, J.H.; Kruse, M.L.; Stoll, M.; Unger, T. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscl. Thromb. Vasc. Biol. 2005, 25, 57–64.
- AbdAlla, S.; Lother, H.; Langer, A.; el Faramawy, Y.; Quitterer, U. Factor XIIIA Transglutaminase Crosslinks AT1 Receptor Dimers of Monocytes at the Onset of Atherosclerosis. Cell 2004, 119, 343–354.
- Miura, S.; Karnik, S.S.; Saku, K. Constitutively Active Homo-oligomeric Angiotensin II Type 2 Receptor Induces Cell Signaling Independent of Receptor Conformation and Ligand Stimulation. J. Biol. Chem. 2005, 280, 18237–18244.
- Porrello, E.R.; Pfleger, K.D.G.; Seeber, R.M.; Qian, H.; Oro, C.; Abogadie, F.; Delbridge, L.M.D.; Thomas, W.G. Heteromerization of angiotensin receptors changes trafficking and arrestin recruitment profiles. Cell. Signal. 2011, 23, 1767–1776.
- Abadir, P.M.; Carey, R.M.; Siragy, H.M. Angiotensin AT2 Receptors Directly Stimulate Renal Nitric Oxide in Bradykinin B2-Receptor–Null Mice. Hypertension 2003, 42, 600–604.
- Abadir, P.M.; Periasamy, A.; Carey, R.M.; Siragy, H.M. Angiotensin II Type 2 Receptor–Bradykinin B2 Receptor Functional Heterodimerization. Hypertension 2006, 48, 316–322.
- Patel, S.N.; Ali, Q.; Samuel, P.; Steckelings, U.M.; Hussain, T. Angiotensin II Type 2 Receptor and Receptor Mas Are Colocalized and Functionally Interdependent in Obese Zucker Rat Kidney. Hypertension 2017, 70, 831–838.
- Walters, P.E.; Gaspari, T.A.; Widdop, R.E. Angiotensin-(1–7) Acts as a Vasodepressor Agent Via Angiotensin II Type 2 Receptors in Conscious Rats. Hypertension 2005, 45, 960–966.
- Durand, M.J.; Raffai, G.; Weinberg, B.D.; Lombard, J.H. Angiotensin-(1-7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1024–H1033.
- Roks, A.J.; Nijholt, J.; van Buiten, A.; van Gilst, W.H.; de Zeeuw, D.; Henning, R.H. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1-7) on angiotensin II. J. Hypertens. 2004, 22, 2355–2361.
- AbdAlla, S.; Lother, H.; Abdel-tawab, A.M.; Quitterer, U. The Angiotensin II AT2 Receptor Is an AT1Receptor Antagonist. J. Biol. Chem. 2001, 276, 39721–39726.
- Inuzuka, T.; Fujioka, Y.; Tsuda, M.; Fujioka, M.; Satoh, A.O.; Horiuchi, K.; Nishide, S.; Nanbo, A.; Tanaka, S.; Ohba, Y. Attenuation of ligand-induced activation of angiotensin II type 1 receptor signaling by the type 2 receptor via protein kinase C. Sci Rep 2016, 6, 21613.
- Rivas-Santisteban, R.; Rodriguez-Perez, A.I.; Muñoz, A.; Reyes-Resina, I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson’s disease that increases with levodopa-induced dyskinesia. J. Neuroinflamm. 2020, 17, 243.
- Lanctôt, P.M.; Leclerc, P.C.; Escher, E.; Leduc, R.; Guillemette, G. Role of N-glycosylation in the expression and functional properties of human AT1 receptor. Biochemistry 1999, 38, 8621–8627.
- Lanctot, P.M.; Leclerc, P.C.; Clément, M.; Auger-Messier, M.; Escher, E.; Leduc, R.; Guillemette, G. Importance of N-glycosylation positioning for cell-surface expression, targeting, affinity and quality control of the human AT1 receptor. Biochem. J. 2005, 390, 367–376.
- Qian, H. Association of -Arrestin 1 with the Type 1A Angiotensin II Receptor Involves Phosphorylation of the Receptor Carboxyl Terminus and Correlates with Receptor Internalization. Mol. Endocrinol. 2001, 15, 1706–1719.
- Kule, C.E.; Karoor, V.; Day, J.N.E.; Thomas, W.G.; Baker, K.M.; Dinh, D.; Acker, K.A.; Booz, G.W. Agonist-dependent internalization of the angiotensin II type one receptor (AT1): Role of C-terminus phosphorylation in recruitment of β-arrestins. Regul. Pept. 2004, 120, 141–148.
- Chen, K.; Fu, C.; Chen, C.; Jose, P.A.; Zeng, C. Role of GRK4 in the Regulation of Arterial AT1 Receptor in Hypertension. J. Am. Soc. Hypertens. 2016, 10, e3.
- Olivares-Reyes, J.A.; Smith, R.D.; Hunyady, L.; Shah, B.H.; Catt, K.J. Agonist-induced Signaling, Desensitization, and Internalization of a Phosphorylation-deficient AT1A Angiotensin Receptor. J. Biol. Chem. 2001, 276, 37761–37768.
- Leclerc, P.C.; Lanctot, P.M.; Auger-Messier, M.; Escher, E.; Leduc, R.; Guillemette, G. S-nitrosylation of cysteine 289 of the AT1 receptor decreases its binding affinity for angiotensin II: S -nitrosylation of the AT1 receptor. Br. J. Pharmacol. 2006, 148, 306–313.
- Sica, D.A.; Gehr, T.W.B.; Ghosh, S. Clinical Pharmacokinetics of Losartan. Clin. Pharmacokinet. 2005, 44, 797–814.
- Barber, M.N.; Sampey, D.B.; Widdop, R.E. AT2 Receptor Stimulation Enhances Antihypertensive Effect of AT1 Receptor Antagonist in Hypertensive Rats. Hypertension 1999, 34, 1112–1116.
- Violin, J.D.; DeWire, S.M.; Yamashita, D.; Rominger, D.H.; Nguyen, L.; Schiller, K.; Whalen, E.J.; Gowen, M.; Lark, M.W. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J. Pharmacol. Exp. Ther. 2010, 335, 572–579.
- Cui, Y.; Kassmann, M.; Nickel, S.; Zhang, C.; Alenina, N.; Anistan, Y.M.; Schleifenbaum, J.; Bader, M.; Welsh, D.G.; Huang, Y.; et al. Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor. J. Am. Heart Assoc. 2022, 11, e022070.
- Jean-Charles, P.-Y.; Kaur, S.; Shenoy, S.K. G Protein–Coupled Receptor Signaling Through β-Arrestin–Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158.
- Ma, Z.; Viswanathan, G.; Sellig, M.; Jassal, C.; Choi, I.; Garikipati, A.; Xiong, X.; Nazo, N.; Rajagopal, S. β-Arrestin–Mediated Angiotensin II Type 1 Receptor Activation Promotes Pulmonary Vascular Remodeling in Pulmonary Hypertension. JACC Basic Transl. Sci. 2021, 6, 854–869.
- Boerrigter, G.; Soergel, D.G.; Lark, M.W.; Burnett, J.C. TRV120027, a Novel Beta-Arrestin Biased Ligand at the Angiotensin II Type I Receptor, Unloads the Heart and Maintains Renal Function When Added to Furosemide in Experimental Heart Failure. J. Card. Fail. 2011, 17, S63–S64.
- Kanki, H.; Sasaki, T.; Matsumura, S.; Yokawa, S.; Yukami, T.; Shimamura, M.; Sakaguchi, M.; Furuno, T.; Suzuki, T.; Mochizuki, H. β-arrestin-2 in PAR-1-biased signaling has a crucial role in endothelial function via PDGF-β in stroke. Cell Death Dis. 2019, 10, 100.





