Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | GHIZLANE BENDRISS | -- | 2700 | 2023-08-23 09:07:02 | | | |
| 2 | Dean Liu | -5 word(s) | 2695 | 2023-08-24 03:23:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bendriss, G.; Macdonald, R.; Mcveigh, C. Microbial Reprogramming in Obsessive–Compulsive Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/48353 (accessed on 07 February 2026).
Bendriss G, Macdonald R, Mcveigh C. Microbial Reprogramming in Obsessive–Compulsive Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/48353. Accessed February 07, 2026.
Bendriss, Ghizlane, Ross Macdonald, Clare Mcveigh. "Microbial Reprogramming in Obsessive–Compulsive Disorders" Encyclopedia, https://encyclopedia.pub/entry/48353 (accessed February 07, 2026).
Bendriss, G., Macdonald, R., & Mcveigh, C. (2023, August 23). Microbial Reprogramming in Obsessive–Compulsive Disorders. In Encyclopedia. https://encyclopedia.pub/entry/48353
Bendriss, Ghizlane, et al. "Microbial Reprogramming in Obsessive–Compulsive Disorders." Encyclopedia. Web. 23 August, 2023.
Copy Citation
Obsessive–compulsive disorder (OCD) is a debilitating mental health disorder characterized by intrusive thoughts (obsessions) and repetitive behaviors (compulsions). Dysbiosis, an imbalance in the gut microbial composition, has been associated with various health conditions, including mental health disorders, autism, and inflammatory diseases. While the exact mechanisms underlying OCD remain unclear, researchers present a growing body of evidence suggesting a potential link between dysbiosis and the multifaceted etiology of OCD, interacting with genetic, neurobiological, immunological, and environmental factors.
OCD
obsessive–compulsive disorder
microbiota
gut
gut–brain axis
probiotics
fecal transplants
1. Introduction
Obsessive–compulsive Disorder (OCD) is a chronic mental health disorder characterized by the presence of intrusive and persistent thoughts that cause distress called obsessions; these are followed by compulsions, which are repetitive behaviors or mental acts that individuals feel driven to perform to calm their obsessions [1]. OCD affects approximately 2–3% of the global population and greatly interferes with quality of life, disturbing the daily functioning of an individual, from eating to bathing to walking or even breathing. For example, an individual with OCD can spend thirty minutes closing a door and verifying it is closed, with the hope that the anxiety might be calmed after a certain number of repetitions. The decision making of individuals with OCD is greatly affected, as every decision may be felt as a threat, leading to maximum indecisiveness [2][3][4]. It is a very debilitating disorder that often hides behind another disease or disorder. Indeed, although OCD is now recognized as an independent disorder category, it often occurs with other disorders such as autism, attention deficit hyperactivity disorder (ADHD), depression, general anxiety disorder, eating disorder, hoarding disorder, Tourette syndrome, panic disorder, or schizophrenia [5]. This category includes other disorders such as hoarding disorder, hair-pulling disorder, and skin-picking disorder [6].
The exact mechanisms underlying OCD are not yet fully understood. Research has highlighted several associations, leading to the conclusion that a combination of genetic, neurobiological, immunological, and environmental factors may contribute to its development. Indeed, studies have identified the heritability of OCD through multiple genes such as the serotonin transporter gene (SLC6A4) and the gene encoding the dopamine D2 receptor (DRD2) [7][8]. Also, OCD has been associated with neurobiological changes such as the dysregulation of the cortico-striato-thalamo-cortical (CSTC) circuit. Brain regions involved include the orbitofrontal cortex, the anterior cingulate cortex, and the basal ganglia, as well as dysregulation in neurotransmitters like serotonin, dopamine, and glutamate [6][9][10][11]. In addition, environmental factors, such as childhood trauma, including physical and/or sexual abuse, have been associated with an increased risk of developing OCD. Finally, stressful life events, such as significant life changes or trauma, have been found to precede the onset or exacerbation of OCD symptoms [6][12].
To date, cognitive behavioral therapy (CBT) and pharmacotherapy are the primary treatments for OCD [13][14]. CBT typically involves exposure and response prevention, where individuals are gradually encouraged to face their obsessions while refraining from engaging in their compulsive behaviors. This helps to reduce the anxiety associated with the obsessions and weaken the link between the obsession and compulsion. CBT has been shown to be effective in reducing OCD symptoms and improving overall functioning [14]. On the other hand, selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, sertraline, and fluvoxamine, are the first-line medications for OCD treatment [13][15]. These medications increase serotonin levels in the brain and help alleviate symptoms. Additionally, combining SSRIs with antipsychotics or glutamate modulators is sometimes used for individuals who do not respond adequately to SSRIs alone [16]. Despite the availability of treatment options for OCD, there are significant limitations that warrant the exploration of novel therapeutic approaches. While these interventions can be effective for some individuals, many patients experience only partial responses to treatment, lingering symptoms, and high rates of relapse [16][17]. CBT is efficient, but each treatment plan is specific to an obsession and does not avoid the appearance of another obsession and compulsion later, which would require another course of CBT. Additionally, there are side effects associated with the use of medication, such as gastrointestinal disturbances and sexual dysfunction, which can further impact treatment adherence and quality of life [18].
The limitations of current treatment options emphasize the need for innovative and therapeutic approaches that target the etiology of OCD. To date, several factors have been proposed to contribute to the development of OCD, and it is difficult to point to one single cause. Nevertheless, there is one emerging avenue of investigation that presents itself as promising and key for the understanding and treatment of OCD: the gut microbiota. The gut microbiota comprises trillions of microorganisms residing in the gastrointestinal tract, from bacteria to fungi to viruses, archaea, and protozoa. These microbes outnumber human cells by a factor of 10, and the genes they express form the microbiome [19]. These are usually classified into three categories according to their interaction with their human hosts: beneficial, pathogens, and commensal microbes. Because they control each other’s growth, eubiosis (the undefined but balanced composition of the gut microbiota) is essential to prevent the overgrowth of pathogens or a lack of growth of certain beneficial microbes from lacking. In contrast, dysbiosis refers to an imbalance in the composition or function of the gut microbiome. It can occur when there are changes in the relative abundance of certain microbial species or alterations in the overall diversity, resulting in the alteration of the metabolites produced by the microbiota. Dysbiosis has been associated with various health conditions, including metabolic disorders, mental health disorders, autoimmune diseases, and inflammatory bowel diseases [20][21][22][23][24][25][26][27][28][29][30][31][32][33]. Interestingly, dysbiosis has been associated with all disorders where OCD has been found as a comorbidity such as autism, Tourette Syndrome, anxiety disorders, panic disorder, eating disorders, depression, and hoarding disorder but also gastrointestinal diseases such as ulcerative colitis and Crohn’s disease [34][35][36].
2. Mechanisms of the Microbiota–Gut–Brain Axis (MGBA)
The MGBA refers to the bidirectional communication between the gut microbiota, the gastrointestinal tract, and the central nervous system (CNS). From brain to gut, endocrine systems such as the hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes regulate the gut microbiota [37][38][39]. From gut to brain, the gut microbiome, consisting of microbes, their genomes, and their products, can influence brain function through a variety of mechanisms, summarized in Figure 1. Researchers will describe these below as (1) the endocrine pathway; (2) the nervous pathway; and (3) the immune pathway.
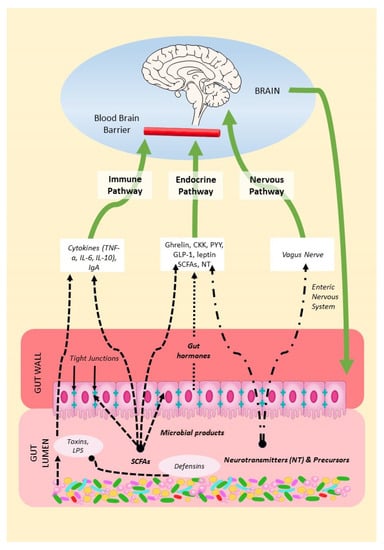
Figure 1. Mechanisms of the MGBA. Gut microbes in the gut lumen produce SCFAs, neurotransmitters, precursors, and defensins but also toxins. Microbial products can thus modulate gut permeability and immune function, endocrine secretions, and the vagal function of the brain.
3. Microbial Reprogramming Strategies
3.1. Prebiotics, Probiotics, and Postbiotics
If dysbiosis is a central element in the development of OCD, then it would be expected that the manipulation of the gut microbiota might influence its occurrence and offer potential options for its treatment. This does in fact appear to be the case, researhers gather clinical evidence of the use of probiotics and fecal microbiota transplants in the treatment of OCD.
A probiotic is a live organism that, when ingested in adequate amounts, exerts a health benefit on the host [40]. Probiotics use dietary fibers or resistant starch as nutrient sources (or prebiotics) to produce beneficial metabolites (postbiotics). The term synbiotic is used to refer to the mixture of both prebiotics and probiotics [41]. Dietary fibers and resistant starch, therefore, play an essential role in fermentation and postbiotic production [42][43][44]. Westernized diets are characterized by a relatively low intake of dietary fiber, which could explain the presence of dysbiosis in most modern diseases and disorders. Dietary fibers also include plant-based carbohydrates, such as polyphenols, and non-carbohydrate compounds, such as lignin. Probiotics such as Lactobacillus, Bifidobacterium, and Akkermansia can use these compounds to produce SCFAs, which, in turn, promote various beneficial effects in the host [45][46][47][48][49][50][51].
Over the last decade, a number of studies have shown promising results for the use of probiotics in the treatment of OCD. However, while a growing number of studies have investigated the potential value of probiotics in treating autism and ADHD, investigations of probiotic interventions for OCD are still at their very early stages, with most studies being performed on animal models.
Kantak et al. (2014) found that two-week pretreatments with Lactobacillus rhamnosus GG had the ability to reduce obsessive–compulsive disorder in mice. The results were comparable to treatment with fluoxetine [51].
In 2018, Tabouy et al., using Shank3 KO mice (a model used to study neurodevelopmental disorders such as autism), found Lactobacillus reuteri to be in a decreased relative abundance in the Shank3 KO. The treatment of Shank3 KO mice with Lactobacillus reuteri induced a significant decrease in repetitive behaviors in both males and females [52].
In a study conducted by Szklany et al. (2020), male mice receiving (from the day of birth onwards) a prebiotic mixture composed of short-chain galactooligosaccharides (scGOS) and long-chain fructo-oligosaccharide (lcFOS) exhibited changes in the serotonergic system [53]. These neurological modulations were associated with behavioral changes, such as a reduction in anxiety and repetitive behavior during development and increased social interest in adulthood compared with mice fed a control diet. The brains of the treated group exhibited altered mRNA expression in astrocytic glial fibrillary acidic protein and microglial integrin alpha M. There was also enhanced mRNA expression in BDNF in the prefrontal cortex. Additionally, analysis of the cecal content of the treated animals revealed relatively increased levels of SCFA, such as butyric acid, and decreased levels of valeric, isobutyric, and isovaleric acid [53].
Another animal study conducted by Sanikhani et al. (2020) demonstrated the effectiveness of Lactobacillus casei Shirota in treating OCD in a rat model. After daily administrations of L. casei Shirota (109 CFU/mL for four weeks), the probiotic showed beneficial effects, possibly effected through the modulation of genes related to serotonin. Following concurrent treatment with L. casei Shirota and fluoxetine, the expression level of Bdnf significantly increased, while the expression of Htr2a (serotonin receptor 2A) decreased in the orbitofrontal cortex tissues of all rats involved in the study [54].
In 2021, Sunand et al. [55] found that selected probiotic strains and complex treatments with probiotics significantly ameliorated microbial diversity; repetitive behaviors; and the concentrations of NF-a, BDNF, and 5-HT.
In 2022, Alghamdi et al. [56], using an animal model of autism induced by propionic acid, found that subjects with cognitive dysfunction had altered levels of neurotransmitters in their brains. However, in the group of animals treated with probiotics, neurotransmitter levels were 1.2-fold higher compared with the control group. In the same study, the alpha-melanocyte-stimulating hormone (α-MSH) was monitored. α-MSH acts on melanocortin type 4 receptors (MC4R), a receptor that interacts with neurochemical systems that regulate socioemotional behaviors, including oxytocin and dopamine. Oxytocin can influence social cognition by modulating various neurochemical systems, including serotonin, glutamate, dopamine, and GABA neurotransmitters in specific brain regions, such as the hypothalamus, amygdala, and hippocampus. The study observed significantly lower levels of α-MSH in animals treated with propionic acid compared with the controls. However, this effect was reversed by the administration of bee pollen and a mixed probiotic bacteria preparation called ProtexinR, which contains beneficial bacteria such as Bifidobacterium infantis, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus rhamnosus, and Streptococcus thermophiles. The concentration of the mixed probiotic bacteria in ProtexinR was 1 billion CFU per gram [56].
Pochakom (2022) investigated supplementation with Lacticaseibacillus rhamnosus HA-11 (Lr) and Ligilactobacillus salivarius HA-118 (Ls) in the BTBR T+ Itpr3tf/J (BTBR) mouse model of autism (109 CFU/mL in drinking water for 4 weeks) [57]. Supplementation with Lr, but not Ls, increased the microbial richness and diversity and increased the concentrations of beneficial neuroactive compounds, such as 5-aminovaleric acid and choline. Both the Lr and Ls treatments reduced behavioral deficits in social novelty preference, but no changes in hyperactivity or repetitive behaviors were observed [57]. This suggests that not all probiotic microbes result in the same outcomes, and a more complex mix of microbes might actually be required to target various behaviors.
Sen et al. 2022 [58] found that the daily oral administration of Blautia stercoris MRx0006 attenuated social and repetitive behaviors in a mouse model of autism. The study showed that MRx0006 increases the expression of oxytocin and its receptor in hypothalamic cells in vitro and hypothalamic oxytocin mRNA in mice while altering the metabolome profile. It was proposed that biotherapy using Blautia stercoris would be a viable treatment option for autism.
Studies on human subjects are rarer but encouraging. A double-blind randomized controlled trial focused on the effect of a synbiotic called Synbiotic 2000, composed of three anti-inflammatory lactic acid bacteria and four anti-inflammatory fibers, on patients with ADHD [40]. One of the measured outcomes was repetitive behavior. Synbiotic 2000 reduced both the total score of autism symptoms and restricted, repetitive, and stereotyped behaviors, as compared with a placebo [52]. Similarly, in a case report on a child with autism, Sacharomyces boulardii was shown to reduce OCD behavior [59].
3.2. Fecal Microbiota Transplants
Fecal microbiota transplantation (FMT), or the transfer of fecal matter from a healthy donor to a patient, has emerged as another promising therapeutic approach for restoring a healthy gut microbiome and achieving beneficial effects in various diseases [60]. Currently, there are no FMT studies that have been performed specifically to treat OCD. However, several studies have noted significant changes in microbial ecology, metabolism, and behavior observed in patients after FMT, most of them providing strong support for FMT as a therapeutic method to treat OCD [61][62][63][64][65][66].
Kang et al. (2017) published an important follow-up after the publication of the first clinical trial results using FMT on autistic children [67]. Spectacular improvements were observed in GI symptoms, autism-related symptoms, and gut microbiota diversity with a higher abundance of Bifidobacteria and Prevotella, and these were sustained after two years [61]. The autism-related symptoms even exhibited further improvement, suggesting that the fecal transplants might have initiated further changes during the two-year period. These findings underscore the long-term safety and effectiveness of FMT as a potential therapy for gut-dysbiosis-associated disorders. Although the focus of the study was not OCD behavior, it is particularly relevant considering the close relationship between gut dysbiosis and brain dysfunction. For example, Kilinçarslan et al. (2020) found that the severity of several factors, including obsession, decreased after FMT in patients with inflammatory bowel disease [68]. This suggests that the restoration of a healthy gut microbial community through FMT can have positive effects on psychological symptoms associated with certain diseases. Alghamdi et al. (2022) conducted a study in a rodent model of autism and included the use of FMT from healthy donor rats, which resulted in a significant increase in α-MSH levels by 2.7 fold (compared with 1.2 fold for probiotics) and an increase in the brain levels of neurotransmitters (1.6 fold) and substance P (2.2 fold) to above that of the controls [56]. These results suggest that FMT might be superior to probiotics in initiating metabolic changes; however, further clinical studies are needed to compare both the efficacy and safety of FMT and probiotics.
Although not focusing on OCD but on autism, a very interesting recent study by Wang et al. (2023) highlights important changes after fecal transplants [69]. Fecal microbiota samples from ASD children and healthy donors were transplanted into a mouse model of ASD. The researchers conducted 16S rRNA gene sequencing on fecal samples and untargeted metabolomic analysis on samples to identify differences in gut microbial communities and metabolic pathways related to ASD behaviors. mRNA sequencing analysis was also performed on colon and brain tissues after sacrificing the animals to identify enriched signaling pathways and potential molecular mechanisms. The study revealed metabolite changes related to serotonergic and glutamatergic synapse pathways. They also demonstrated that these were associated with behavioral changes in ASD: there was an increase in ASD-like behaviors in mice that received FMT from ASD donors but a decrease in such behaviors in mice that received FMT from healthy donors. Indeed, the colonization of certain bacterial genera, such as Bacteroides, Odoribacter, Turicibacter, and Alistipes, was correlated with an improvement in behavior after FMT, but this did not specifically point to OCD behavior. However, the changes in serotoninergic and glutamatergic pathways might also predict positive outcomes for future OCD studies.
The close link between gut dysbiosis and brain function underscores the importance of targeting the gut microbiota for therapeutic interventions. FMT offers a unique opportunity to restore a healthy gut microbial community and potentially alleviate symptoms associated with various disorders. These findings highlight the potential benefits of FMT in improving mental health conditions and support the further exploration of this therapeutic approach.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; p. 947.
- Oren, E.; Dar, R.; Liberman, N. Obsessive-compulsive tendencies are related to a maximization strategy in making decisions. Front. Psychol. 2018, 9, 778.
- Nestadt, G.; Kamath, V.; Maher, B.S.; Krasnow, J.; Nestadt, P.; Wang, Y.; Bakker, A.; Samuels, J. Doubt and the decision-making process in obsessive-compulsive disorder. Med. Hypotheses 2016, 96, 1–4.
- Pushkarskaya, H.; Tolin, D.; Ruderman, L.; Kirshenbaum, A.; Kelly, J.M.; Pittenger, C.; Levy, I. Decision-making under uncertainty in obsessive-compulsive disorder. J. Psychiatr. Res. 2015, 69, 166–173.
- Murphy, D.L.; Timpano, K.R.; Wheaton, M.G.; Greenberg, B.D.; Miguel, E.C. Obsessive-compulsive disorder and its related disorders: A reappraisal of obsessive-compulsive spectrum concepts. Dialogues Clin. Neurosci. 2010, 12, 131–148.
- Jalal, B.; Chamberlain, S.R.; Sahakian, B.J. Obsessive-compulsive disorder: Etiology, neuropathology, and cognitive dysfunction. Brain Behav. 2023, 13, e3000.
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641.
- Mattheisen, M.; Samuels, J.F.; Wang, Y.; Greenberg, B.D.; Fyer, A.J.; McCracken, J.T.; Geller, D.A.; Murphy, D.L.; Knowles, J.A.; Grados, M.A.; et al. Genome-wide association study in obsessive-compulsive disorder: Results from the OCGAS. Mol. Psychiatry 2015, 20, 337–344.
- Frick, L.; Pittenger, C. Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. J. Immunol. Res. 2016, 2016, 8606057.
- Mahjani, B.; Bey, K.; Boberg, J.; Burton, C. Genetics of obsessive-compulsive disorder. Psychol. Med. 2021, 51, 2247–2259.
- Purty, A.; Nestadt, G.; Samuels, J.F.; Viswanath, B. Genetics of obsessive-compulsive disorder. Indian J. Psychiatry 2019, 61, S37–S42.
- Fawcett, E.J.; Power, H.; Fawcett, J.M. Women are at greater risk of OCD than men: A meta-analytic review of OCD prevalence worldwide. J. Clin. Psychiatry 2020, 81, 13075.
- Grassi, G.; Cecchelli, C.; Vignozzi, L.; Pacini, S. Investigational and experimental drugs to treat obsessive-compulsive disorder. J. Exp. Pharmacol. 2020, 12, 695–706.
- Foa, E.B. Cognitive behavioral therapy of obsessive-compulsive disorder. Dialogues Clin. Neurosci. 2010, 12, 199–207.
- Kellner, M. Drug treatment of obsessive-compulsive disorder. Dialogues Clin. Neurosci. 2010, 12, 187–197.
- Fineberg, N.A.; Gale, T.M.; Sivakumaran, T. A review of antipsychotics in the treatment of obsessive compulsive disorder. J. Psychopharmacol. 2006, 20, 97–103.
- Eisen, J.L.; Goodman, W.K.; Keller, M.B.; Warshaw, M.G.; DeMarco, L.M.; Luce, D.D.; Rasmussen, S.A. Patterns of remission and relapse in obsessive-compulsive disorder: A 2-year prospective study. J. Clin. Psychiatry 1999, 60, 346–351.
- Ferguson, J.M. SSRI antidepressant medications: Adverse effects and tolerability. Prim Care Companion J. Clin. Psychiatry 2001, 3, 22–27.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836.
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm. Bowel Dis. 2012, 18, 1092–1100.
- Kang, S.; Denman, S.E.; Morrison, M.; Yu, Z.; Dore, J.; Leclerc, M.; McSweeney, C.S. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010, 16, 2034–2042.
- Hou, M.; Xu, G.; Ran, M.; Luo, W.; Wang, H. APOE-ε4 carrier status and gut microbiota dysbiosis in patients with Alzheimer disease. Front. Neurosci. 2021, 15, 619051.
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016, 74, 624–634.
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between gut and brain in Alzheimer’s disease: The role of gut microbiota modulation strategies. Nutrients 2021, 13, 690.
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358.
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in Autism: Plausible Role of Maternal Inflammation, Dietary Omega 3, and Microbiota. Neural. Plast. 2016, 2016, 3597209.
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213.
- Li, Q.; Zhou, J.M. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016, 324, 131–139.
- Ding, H.T.; Taur, Y.; Walkup, J.T. Gut microbiota and autism: Key concepts and findings. J. Autism. Dev. Disord. 2017, 47, 480–489.
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918.
- Frazier, T.H.; DiBaise, J.K.; McClain, C.J. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J. Parenter. Enteral. Nutr. 2011, 35, 14S–20S.
- Musso, G.; Gambino, R.; Cassader, M. Obesity, diabetes, and gut microbiota: The hygiene hypothesis expanded? Diabetes Care 2010, 33, 2277–2284.
- Jess, T. Microbiota, antibiotics, and obesity. N. Engl. J. Med. 2014, 371, 2526–2528.
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987.
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050.
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238.
- Sudo, N. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv. Exp. Med. Biol. 2014, 817, 177–194.
- de Weerth, C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017, 83, 458–471.
- Sudo, N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem. Immunol. Allergy 2012, 98, 163–175.
- Skott, E.; Yang, L.L.; Stiernborg, M.; Söderström, Å.; Rȕegg, J.; Schalling, M.; Forsell, Y.; Giacobini, M.; Lavebratt, C. Effects of a synbiotic on symptoms, and daily functioning in attention deficit hyperactivity disorder—A double-blind randomized controlled trial. Brain Behav. Immun. 2020, 89, 9–19.
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156.
- Wu, Z.; Xu, Q.; Wang, Q.; Chen, Y.; Lv, L.; Zheng, B.; Yan, R.; Jiang, H.; Shen, J.; Wang, S.; et al. The impact of dietary fibers on Clostridioides difficile infection in a mouse model. Front. Cell. Infect. Microbiol. 2022, 12, 1028267.
- Chen, K.; Chen, H.; Faas, M.M.; de Haan, B.J.; Li, J.; Xiao, P.; Zhang, H.; Diana, J.; de Vos, P.; Sun, J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017, 61, 1601006.
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071.
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721.
- Demirci, M.; Tokman, H.B.; Uysal, H.K.; Demiryas, S.; Karakullukcu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B.S. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019, 47, 365–371.
- Klaenhammer, T.R.; Altermann, E.; Pfeiler, E.; Buck, B.L.; Goh, Y.J.; O’Flaherty, S.; Barrangou, R.; Duong, T. Functional genomics of probiotic Lactobacilli. J. Clin. Gastroenterol. 2008, 42 Pt 2 (Suppl. 3), S160–S162.
- Maassen, C.B.; Claassen, E. Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine 2008, 26, 2056–2057.
- Martín, R.; Delgado, S.; Maldonado, A.; Jiménez, E.; Olivares, M.; Fernández, L.; Sobrino, O.J.; Rodríguez, J.M. Isolation of lactobacilli from sow milk and evaluation of their probiotic potential. J. Dairy Res. 2009, 76, 418–425.
- Moorthy, G.; Murali, M.R.; Devaraj, S.N. Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition 2009, 25, 350–358.
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79.
- Tabouy, L.; Getselter, D.; Ziv, O.; Karpuj, M.; Tabouy, T.; Lukic, I.; Maayouf, R.; Werbner, N.; Ben-Amram, H.; Nuriel-Ohayon, M.; et al. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorers. Brain Behav. Immun. 2018, 73, 310–319.
- Szklany, K.; Wopereis, H.; de Waard, C.; van Wageningen, T.; An, R.; van Limpt, K.; Knol, J.; Garssen, J.; Knippels, L.M.J.; Belzer, C.; et al. Supplementation of dietary non-digestible oligosaccharides from birth onwards improve social and reduce anxiety-like behaviour in male BALB/c mice. Nutr. Neurosci. 2020, 23, 896–910.
- Sanikhani, N.S.; Modarressi, M.H.; Jafari, P.; Vousooghi, N.; Shafei, S.; Akbariqomi, M.; Heidari, R.; Lavasani, P.S.; Yazarlou, F.; Motevaseli, E.; et al. The Effect of Lactobacillus casei consumption in improvement of obsessive-compulsive disorder: An animal study. Probiotics Antimicrob. Proteins 2020, 12, 1409–1419.
- Sunand, K.; Mohan, G.K.; Bakshi, V. Enrichment of gut ecosystem by daily supplementation of selective probiotic strains and probiotic complex in dysbiosis condition of autism. Int. J. Pharm. Res. 2021, 13, 4614–4628.
- Alghamdi, M.A.; Al-Ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-Ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562.
- Pochakom, A.; Mu, C.; Rho, J.M.; Tompkins, T.A.; Mayengbam, S.; Shearer, J. Selective probiotic treatment positively modulates the microbiota-gut-brain axis in the BTBR mouse model of autism. Brain Sci 2022, 12, 781.
- Sen, P.; Sherwin, E.; Sandhu, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Golubeva, A.; Fitzgerald, P.; Paula Ventura Da Silva, A.; Chruścicka-Smaga, B.; Olavarría-Ramírez, L.; et al. The live biotherapeutic Blautia stercoris MRx0006 attenuates social deficits, repetitive behaviour, and anxiety-like behaviour in a mouse model relevant to autism. Brain Behav. Immun. 2022, 106, 115–126.
- Kobliner, V.; Mumper, E.; Baker, S.M. Reduction in Obsessive Compulsive Disorder and self-injurious behavior with Saccharomyces boulardii in a child with autism: A case report. Integr. Med. 2018, 17, 38–41.
- Al-Ali, D.; Ahmed, A.; Shafiq, A.; McVeigh, C.; Chaari, A.; Zakaria, D.; Bendriss, G. Fecal microbiota transplants: A review of emerging clinical data on applications, efficacy, and risks (2015–2020). Qatar Med. J. 2021, 2021, 5.
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821.
- Ananthaswamy, A. Faecal transplant eases symptoms of Parkinson’s disease. New Sci. 2011, 209, 8–9.
- Ianiro, G.; Segal, J.P.; Mullish, B.H.; Quraishi, M.N.; Porcari, S.; Fabiani, G.; Gasbarrini, A.; Cammarota, G. Fecal microbiota transplantation in gastrointestinal and extraintestinal disorders. Future Microbiol. 2020, 15, 1173–1183.
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front. Cell. Infect. Microbiol. 2021, 11, 759435.
- Wang, J.; Liu, X.; Li, Q. Interventional strategies for ischemic stroke based on the modulation of the gut microbiota. Front. Neurosci. 2023, 17, 1158057.
- Khanna, S.; Vazquez-Baeza, Y.; González, A.; Weiss, S.; Schmidt, B.; Muñiz-Pedrogo, D.A.; Rainey, J.F., 3rd; Kammer, P.; Nelson, H.; Sadowsky, M.; et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 2017, 5, 55.
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10.
- Kilinçarslan, S.; Evrensel, A. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: An experimental study. Actas Esp. Psiquiatr. 2020, 48, 1–7. Available online: https://www.actaspsiquiatria.es/repositorio//22/123/ENG/22-123-ENG-1-7-952049.pdf (accessed on 9 July 2023).
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl. Psychiatry 2023, 13, 17.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
24 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




