Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by GHIZLANE BENDRISS and Version 2 by Dean Liu.
Obsessive–compulsive disorder (OCD) is a debilitating mental health disorder characterized by intrusive thoughts (obsessions) and repetitive behaviors (compulsions). Dysbiosis, an imbalance in the gut microbial composition, has been associated with various health conditions, including mental health disorders, autism, and inflammatory diseases. While the exact mechanisms underlying OCD remain unclear, this researchersview presents a growing body of evidence suggesting a potential link between dysbiosis and the multifaceted etiology of OCD, interacting with genetic, neurobiological, immunological, and environmental factors.
- OCD
- obsessive–compulsive disorder
- microbiota
- gut
- gut–brain axis
- probiotics
- fecal transplants
1. Introduction
Obsessive–compulsive Disorder (OCD) is a chronic mental health disorder characterized by the presence of intrusive and persistent thoughts that cause distress called obsessions; these are followed by compulsions, which are repetitive behaviors or mental acts that individuals feel driven to perform to calm their obsessions [1]. OCD affects approximately 2–3% of the global population and greatly interferes with quality of life, disturbing the daily functioning of an individual, from eating to bathing to walking or even breathing. For example, an individual with OCD can spend thirty minutes closing a door and verifying it is closed, with the hope that the anxiety might be calmed after a certain number of repetitions. The decision making of individuals with OCD is greatly affected, as every decision may be felt as a threat, leading to maximum indecisiveness [2][3][4][2,3,4]. It is a very debilitating disorder that often hides behind another disease or disorder. Indeed, although OCD is now recognized as an independent disorder category, it often occurs with other disorders such as autism, attention deficit hyperactivity disorder (ADHD), depression, general anxiety disorder, eating disorder, hoarding disorder, Tourette syndrome, panic disorder, or schizophrenia [5]. This category includes other disorders such as hoarding disorder, hair-pulling disorder, and skin-picking disorder [6].
The exact mechanisms underlying OCD are not yet fully understood. Research has highlighted several associations, leading to the conclusion that a combination of genetic, neurobiological, immunological, and environmental factors may contribute to its development. Indeed, studies have identified the heritability of OCD through multiple genes such as the serotonin transporter gene (SLC6A4) and the gene encoding the dopamine D2 receptor (DRD2) [7][8][7,8]. Also, OCD has been associated with neurobiological changes such as the dysregulation of the cortico-striato-thalamo-cortical (CSTC) circuit. Brain regions involved include the orbitofrontal cortex, the anterior cingulate cortex, and the basal ganglia, as well as dysregulation in neurotransmitters like serotonin, dopamine, and glutamate [6][9][10][11][6,9,10,11]. In addition, environmental factors, such as childhood trauma, including physical and/or sexual abuse, have been associated with an increased risk of developing OCD. Finally, stressful life events, such as significant life changes or trauma, have been found to precede the onset or exacerbation of OCD symptoms [6][12][6,12].
To date, cognitive behavioral therapy (CBT) and pharmacotherapy are the primary treatments for OCD [13][14][13,14]. CBT typically involves exposure and response prevention, where individuals are gradually encouraged to face their obsessions while refraining from engaging in their compulsive behaviors. This helps to reduce the anxiety associated with the obsessions and weaken the link between the obsession and compulsion. CBT has been shown to be effective in reducing OCD symptoms and improving overall functioning [14]. On the other hand, selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, sertraline, and fluvoxamine, are the first-line medications for OCD treatment [13][15][13,15]. These medications increase serotonin levels in the brain and help alleviate symptoms. Additionally, combining SSRIs with antipsychotics or glutamate modulators is sometimes used for individuals who do not respond adequately to SSRIs alone [16]. Despite the availability of treatment options for OCD, there are significant limitations that warrant the exploration of novel therapeutic approaches. While these interventions can be effective for some individuals, many patients experience only partial responses to treatment, lingering symptoms, and high rates of relapse [16][17][16,17]. CBT is efficient, but each treatment plan is specific to an obsession and does not avoid the appearance of another obsession and compulsion later, which would require another course of CBT. Additionally, there are side effects associated with the use of medication, such as gastrointestinal disturbances and sexual dysfunction, which can further impact treatment adherence and quality of life [18].
The limitations of current treatment options emphasize the need for innovative and therapeutic approaches that target the etiology of OCD. To date, several factors have been proposed to contribute to the development of OCD, and it is difficult to point to one single cause. Nevertheless, there is one emerging avenue of investigation that presents itself as promising and key for the understanding and treatment of OCD: the gut microbiota. The gut microbiota comprises trillions of microorganisms residing in the gastrointestinal tract, from bacteria to fungi to viruses, archaea, and protozoa. These microbes outnumber human cells by a factor of 10, and the genes they express form the microbiome [19]. These are usually classified into three categories according to their interaction with their human hosts: beneficial, pathogens, and commensal microbes. Because they control each other’s growth, eubiosis (the undefined but balanced composition of the gut microbiota) is essential to prevent the overgrowth of pathogens or a lack of growth of certain beneficial microbes from lacking. In contrast, dysbiosis refers to an imbalance in the composition or function of the gut microbiome. It can occur when there are changes in the relative abundance of certain microbial species or alterations in the overall diversity, resulting in the alteration of the metabolites produced by the microbiota. Dysbiosis has been associated with various health conditions, including metabolic disorders, mental health disorders, autoimmune diseases, and inflammatory bowel diseases [20][21][22][23][24][25][26][27][28][29][30][31][32][33][20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Interestingly, dysbiosis has been associated with all disorders where OCD has been found as a comorbidity such as autism, Tourette Syndrome, anxiety disorders, panic disorder, eating disorders, depression, and hoarding disorder but also gastrointestinal diseases such as ulcerative colitis and Crohn’s disease [34][35][36][34,35,36].
2. Mechanisms of the Microbiota–Gut–Brain Axis (MGBA)
The MGBA refers to the bidirectional communication between the gut microbiota, the gastrointestinal tract, and the central nervous system (CNS). From brain to gut, endocrine systems such as the hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes regulate the gut microbiota [37][38][39][37,38,39]. From gut to brain, the gut microbiome, consisting of microbes, their genomes, and their products, can influence brain function through a variety of mechanisms, summarized in Figure 1. RWesearchers will describe these below as (1) the endocrine pathway; (2) the nervous pathway; and (3) the immune pathway.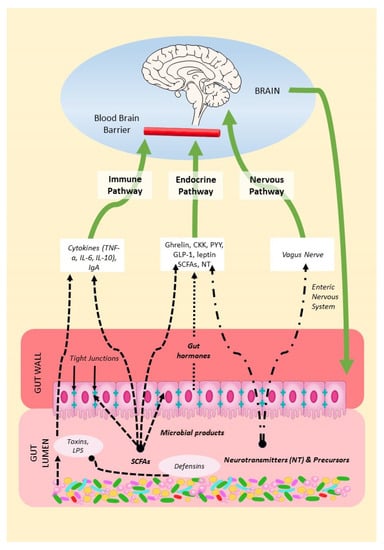
Figure 1. Mechanisms of the MGBA. Gut microbes in the gut lumen produce SCFAs, neurotransmitters, precursors, and defensins but also toxins. Microbial products can thus modulate gut permeability and immune function, endocrine secretions, and the vagal function of the brain.
