
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhenzhen Quan | -- | 3192 | 2023-08-17 14:08:33 | | | |
| 2 | Lindsay Dong | Meta information modification | 3192 | 2023-08-21 03:52:03 | | |
Video Upload Options
Early-life stress during critical periods of brain development can have long-term effects on physical and mental health. Oxytocin is a critical social regulator and anti-inflammatory hormone that modulates stress-related functions and social behaviors and alleviates diseases. Oxytocin-related neural systems show high plasticity in early postpartum and adolescent periods. Early-life stress can influence the oxytocin system long term by altering the expression and signaling of oxytocin receptors. Deficits in social behavior, emotional control, and stress responses may result, thus increasing the risk of anxiety, depression, and other stress-related neuropsychiatric diseases. Oxytocin is regarded as an important target for the treatment of stress-related neuropsychiatric disorders.
1. Introduction
2. The Properties of Oxytocin
3. Oxytocin-Involved Social Behavior
3.1. Positive Social Interactions
3.2. Negative Social Interactions
3.3. Sexual Dimorphism of Oxytocinin Social Behavior
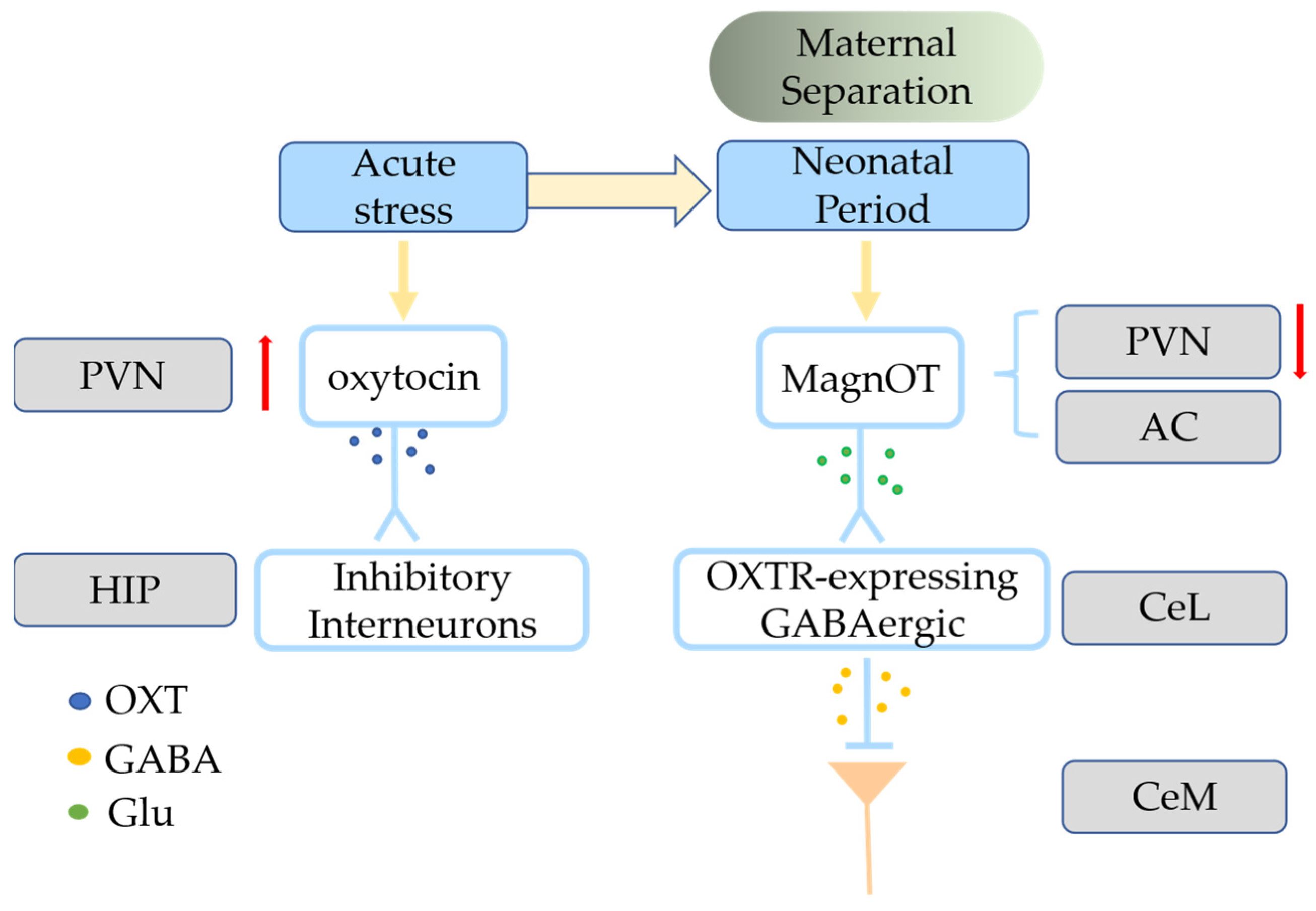
4. Effects of ELS on the Oxytocin System and Central Nervous System
4.1. Effects of ELS on the Oxytocin System
4.2. Abnormalities in Oxytocin System Altered by ELS Correlated with Glial Cells
5. Potential Therapy Strategies of Oxytocin in ELS-Related Neuropsychiatric Disorders
5.1. Autism Spectrum Disorder
5.2. Schizophrenia
5.3. Social Anxiety Disorders
6. Conclusions
References
- Schiavone, S.; Jaquet, V.; Trabace, L.; Krause, K.H. Severe life stress and oxidative stress in the brain: From animal models to human pathology. Antioxid. Redox Signal. 2013, 18, 1475–1490.
- Selye, H. A syndrome produced by diverse nocuous agents. 1936. J. Neuropsychiatry Clin. Neurosci. 1998, 10, 230–231.
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Baker, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118.
- Makris, G.; Eleftheriades, A.; Pervanidou, P. Early Life Stress, Hormones, and Neurodevelopmental Disorders. Horm. Res. Paediatr. 2022, 96, 70–77.
- Wright, M.O.; Crawford, E.; Del Castillo, D. Childhood emotional maltreatment and later psychological distress among college students: The mediating role of maladaptive schemas. Child. Abus. Negl. 2009, 33, 59–68.
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132.
- Colleluori, G.; Galli, C.; Severi, I.; Perugini, J.; Giordano, A. Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link? Cells 2022, 11, 623.
- Love, T.M. The impact of oxytocin on stress: The role of sex. Curr. Opin. Behav. Sci. 2018, 23, 136–142.
- Lang, R.E.; Heil, J.W.; Ganten, D.; Hermann, K.; Unger, T.; Rascher, W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 1983, 37, 314–316.
- García-Cáceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.-X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14.
- George, J.M. Immunoreactive vasopressin and oxytocin: Concentration in individual human hypothalamic nuclei. Science 1978, 200, 342–343.
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006, 135, 7–11.
- Althammer, F.; Grinevich, V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017, 30, e12549.
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2020, 41, 121–145.
- Swanson, L.W.; Sawchenko, P.E.; Wiegand, S.J.; Price, J.L. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980, 198, 190–195.
- Tang, Y.; Benusiglio, D.; Lefevre, A.; Hilfiger, L.; Althammer, F.; Bludau, A.; Hagiwara, D.; Baudon, A.; Darbon, P.; Schimmer, J.; et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020, 23, 1125–1137.
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566.
- Baldi, E.; Costa, A.; Rani, B.; Passani, M.B.; Blandina, P.; Romano, A.; Provensi, G. Oxytocin and Fear Memory Extinction: Possible Implications for the Therapy of Fear Disorders? Int. J. Mol. Sci. 2021, 22, 10000.
- Brownstein, M.J.; Russell, J.T.; Gainer, H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980, 207, 373–378.
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocr. 2004, 25, 150–176.
- Dumais, K.M.; Veenema, A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocr. 2016, 40, 1–23.
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159.
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683.
- Baudon, A.; Clauss Creusot, E.; Althammer, F.; Schaaf, C.P.; Charlet, A. Emerging role of astrocytes in oxytocin-mediated control of neural circuits and brain functions. Prog. Neurobiol. 2022, 217, 102328.
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908.
- Hung, L.W.; Neuner, S.; Polepalli, J.S.; Beier, K.T.; Wright, M.; Walsh, J.J.; Lewis, E.M.; Luo, L.; Deisseroth, K.; Dolen, G.; et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 2017, 357, 1406–1411.
- Oettl, L.L.; Ravi, N.; Schneider, M.; Scheller, M.F.; Schneider, P.; Mitre, M.; da Silva Gouveia, M.; Froemke, R.C.; Chao, M.V.; Young, W.S.; et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90, 609–621.
- Raam, T.; McAvoy, K.M.; Besnard, A.; Veenema, A.H.; Sahay, A. Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat. Commun. 2017, 8, 2001.
- Knoop, M.; Possovre, M.-L.; Jacquens, A.; Charlet, A.; Baud, O.; Darbon, P. The Role of Oxytocin in Abnormal Brain Development: Effect on Glial Cells and Neuroinflammation. Cells 2022, 11, 3899.
- Mehdi, S.F.; Pusapati, S.; Khenhrani, R.R.; Farooqi, M.S.; Sarwar, S.; Alnasarat, A.; Mathur, N.; Metz, C.N.; LeRoith, D.; Tracey, K.J.; et al. Oxytocin and Related Peptide Hormones: Candidate Anti-Inflammatory Therapy in Early Stages of Sepsis. Front. Immunol. 2022, 13, 864007.
- Tang, Y.; Shi, Y.; Gao, Y.; Xu, X.; Han, T.; Li, J.; Liu, C. Oxytocin system alleviates intestinal inflammation by regulating macrophages polarization in experimental colitis. Clin. Sci. (Lond. Engl. 1979) 2019, 133, 1977–1992.
- Huang, S.; Zhu, B.; Cheon, I.S.; Goplen, N.P.; Jiang, L.; Zhang, R.; Peebles, R.S.; Mack, M.; Kaplan, M.H.; Limper, A.H.; et al. PPAR-γ in Macrophages Limits Pulmonary Inflammation and Promotes Host Recovery following Respiratory Viral Infection. J. Virol. 2019, 93, e00030-19.
- Eckertova, M.; Ondrejcakova, M.; Krskova, K.; Zorad, S.; Jezova, D. Subchronic treatment of rats with oxytocin results in improved adipocyte differentiation and increased gene expression of factors involved in adipogenesis. Br. J. Pharmacol. 2011, 162, 452–463.
- Marlin, B.J.; Froemke, R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017, 77, 169–189.
- Borland, J.M.; Grantham, K.N.; Aiani, L.M.; Frantz, K.J.; Albers, H.E. Role of oxytocin in the ventral tegmental area in social reinforcement. Psychoneuroendocrinology 2018, 95, 128–137.
- Walsh, J.J.; Christoffel, D.J.; Malenka, R.C. Neural circuits regulating prosocial behaviors. Neuropsychopharmacology 2022, 48, 79–89.
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e365.
- Song, Z.; Borland, J.M.; Larkin, T.E.; O’Malley, M.; Albers, H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 2016, 74, 164–172.
- Hurlemann, R.; Scheele, D. Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biol. Psychiatry 2016, 79, 185–193.
- Barraza, J.A.; Zak, P.J. Empathy toward strangers triggers oxytocin release and subsequent generosity. Ann. N. Y. Acad. Sci. 2009, 1167, 182–189.
- Walum, H.; Young, L.J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018, 19, 643–654.
- Perkeybile, A.M.; Bales, K.L. Intergenerational transmission of sociality: The role of parents in shaping social behavior in monogamous and non-monogamous species. J. Exp. Biol. 2017, 220, 114–123.
- Insel, T.R.; Shapiro, L.E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA 1992, 89, 5981–5985.
- Bethlehem, R.A.I.; Lombardo, M.V.; Lai, M.C.; Auyeung, B.; Crockford, S.K.; Deakin, J.; Soubramanian, S.; Sule, A.; Kundu, P.; Voon, V.; et al. Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Transl. Psychiatry 2017, 7, e1099.
- Freeman, S.M.; Young, L.J. Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J. Neuroendocrinol. 2016, 28.
- Yamagishi, A.; Lee, J.; Sato, N. Oxytocin in the anterior cingulate cortex is involved in helping behaviour. Behav. Brain Res. 2020, 393, 112790.
- Barchi-Ferreira, A.M.; Osorio, F.L. Associations between oxytocin and empathy in humans: A systematic literature review. Psychoneuroendocrinology 2021, 129, 105268.
- Akinrinade, I.; Kareklas, K.; Teles, M.C.; Reis, T.K.; Gliksberg, M.; Petri, G.; Levkowitz, G.; Oliveira, R.F. Evolutionarily conserved role of oxytocin in social fear contagion in zebrafish. Science 2023, 379, 1232–1237.
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676.
- Menon, R.; Grund, T.; Zoicas, I.; Althammer, F.; Fiedler, D.; Biermeier, V.; Bosch, O.J.; Hiraoka, Y.; Nishimori, K.; Eliava, M.; et al. Oxytocin Signaling in the Lateral Septum Prevents Social Fear during Lactation. Curr. Biol. 2018, 28, 1066–1078.e6.
- Liberzon, I.; Young, E.A. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology 1997, 22, 411–422.
- Matsushita, H.; Latt, H.M.; Koga, Y.; Nishiki, T.; Matsui, H. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience 2019, 417, 1–10.
- Owen, S.F.; Tuncdemir, S.N.; Bader, P.L.; Tirko, N.N.; Fishell, G.; Tsien, R.W. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 2013, 500, 458–462.
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381.
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Triana Del Rio, R.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304.
- Marsh, N.; Marsh, A.A.; Lee, M.R.; Hurlemann, R. Oxytocin and the Neurobiology of Prosocial Behavior. Neuroscientist 2021, 27, 604–619.
- Ferretti, V.; Maltese, F.; Contarini, G.; Nigro, M.; Bonavia, A.; Huang, H.; Gigliucci, V.; Morelli, G.; Scheggia, D.; Managò, F.; et al. Oxytocin Signaling in the Central Amygdala Modulates Emotion Discrimination in Mice. Curr. Biol. 2019, 29, 1938–1953.e6.
- Hasan, M.T.; Althammer, F.; Silva da Gouveia, M.; Goyon, S.; Eliava, M.; Lefevre, A.; Kerspern, D.; Schimmer, J.; Raftogianni, A.; Wahis, J.; et al. A Fear Memory Engram and Its Plasticity in the Hypothalamic Oxytocin System. Neuron 2019, 103, 133–146.e8.
- Oliveira, V.E.M.; Lukas, M.; Wolf, H.N.; Durante, E.; Lorenz, A.; Mayer, A.L.; Bludau, A.; Bosch, O.J.; Grinevich, V.; Egger, V.; et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat. Commun. 2021, 12, 2900.
- Haussler, H.U.; Jirikowski, G.F.; Caldwell, J.D. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J. Chem. Neuroanat. 1990, 3, 271–276.
- Qiao, X.; Yan, Y.; Wu, R.; Tai, F.; Hao, P.; Cao, Y.; Wang, J. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. J. Comp. Physiol. A 2014, 200, 149–159.
- Wang, Y.; Xu, L.; Pan, Y.; Wang, Z.; Zhang, Z. Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis). PLoS ONE 2013, 8, e65807.
- Xu, L.; Pan, Y.; Young, K.A.; Wang, Z.; Zhang, Z. Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton). Neuroscience 2010, 169, 1235–1247.
- Nakajima, M.; Gorlich, A.; Heintz, N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 2014, 159, 295–305.
- Li, K.; Nakajima, M.; Ibanez-Tallon, I.; Heintz, N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.e11.
- Reh, R.K.; Dias, B.G.; Nelson, C.A., 3rd; Kaufer, D.; Werker, J.F.; Kolb, B.; Levine, J.D.; Hensch, T.K. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA 2020, 117, 23242–23251.
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688.
- Biagini, G.; Pich, E.M.; Carani, C.; Marrama, P.; Agnati, L.F. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int. J. Dev. Neurosci. 1998, 16, 187–197.
- Huot, R.L.; Plotsky, P.M.; Lenox, R.H.; McNamara, R.K. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002, 950, 52–63.
- Derks, N.A.; Krugers, H.J.; Hoogenraad, C.C.; Joels, M.; Sarabdjitsingh, R.A. Effects of Early Life Stress on Synaptic Plasticity in the Developing Hippocampus of Male and Female Rats. PLoS ONE 2016, 11, e0164551.
- Danoff, J.S.; Wroblewski, K.L.; Graves, A.J.; Quinn, G.C.; Perkeybile, A.M.; Kenkel, W.M.; Lillard, T.S.; Parikh, H.I.; Golino, H.F.; Gregory, S.G.; et al. Genetic, epigenetic, and environmental factors controlling oxytocin receptor gene expression. Clin. Epigenetics 2021, 13, 23.
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353.
- Loken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548.
- Kojima, S.; Stewart, R.A.; Demas, G.E.; Alberts, J.R. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J. Neuroendocrinol. 2012, 24, 831–840.
- Hertenstein, M.J.; Verkamp, J.M.; Kerestes, A.M.; Holmes, R.M. The communicative functions of touch in humans, nonhuman primates, and rats: A review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006, 132, 5–94.
- Havranek, T.; Lestanova, Z.; Mravec, B.; Strbak, V.; Bakos, J.; Bacova, Z. Oxytocin Modulates Expression of Neuron and Glial Markers in the Rat Hippocampus. Folia Biol. 2017, 63, 91–97.
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69.
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189.
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 529–541.
- Roque, A.; Ochoa-Zarzosa, A.; Torner, L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun. 2016, 55, 39–48.
- Banqueri, M.; Mendez, M.; Gomez-Lazaro, E.; Arias, J.L. Early life stress by repeated maternal separation induces long-term neuroinflammatory response in glial cells of male rats. Stress 2019, 22, 563–570.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487.
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185.
- Perea, G.; Gomez, R.; Mederos, S.; Covelo, A.; Ballesteros, J.J.; Schlosser, L.; Hernandez-Vivanco, A.; Martin-Fernandez, M.; Quintana, R.; Rayan, A.; et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. eLife 2016, 5, e20362.
- Ta, T.T.; Dikmen, H.O.; Schilling, S.; Chausse, B.; Lewen, A.; Hollnagel, J.O.; Kann, O. Priming of microglia with IFN-gamma slows neuronal gamma oscillations in situ. Proc. Natl. Acad. Sci. USA 2019, 116, 4637–4642.
- Hasan, M.; Kanna, M.S.; Jun, W.; Ramkrishnan, A.S.; Iqbal, Z.; Lee, Y.; Li, Y. Schema-like learning and memory consolidation acting through myelination. FASEB J. 2019, 33, 11758–11775.
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123.
- Yamamoto, Y.; Liang, M.; Munesue, S.; Deguchi, K.; Harashima, A.; Furuhara, K.; Yuhi, T.; Zhong, J.; Akther, S.; Goto, H.; et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun. Biol. 2019, 2, 76.
- Zhao, F.; Zhang, H.; Wang, P.; Cui, W.; Xu, K.; Chen, D.; Hu, M.; Li, Z.; Geng, X.; Wei, S. Oxytocin and serotonin in the modulation of neural function: Neurobiological underpinnings of autism-related behavior. Front. Neurosci. 2022, 16, 919890.
- Zhang, R.; Jia, M.X.; Zhang, J.S.; Xu, X.J.; Shou, X.J.; Zhang, X.T.; Li, L.; Li, N.; Han, S.P.; Han, J.S. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: A prospective single-blinded controlled study. Res. Dev. Disabil. 2012, 33, 1136–1146.
- Huber, D.; Veinante, P.; Stoop, R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005, 308, 245–248.
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124.
- Guastella, A.J.; Einfeld, S.L.; Gray, K.M.; Rinehart, N.J.; Tonge, B.J.; Lambert, T.J.; Hickie, I.B. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol. Psychiatry 2010, 67, 692–694.
- El-Ansary, A.; Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J. Neuroinflammation 2012, 9, 265.
- Lee, J.H.; Espinera, A.R.; Chen, D.; Choi, K.E.; Caslin, A.Y.; Won, S.; Pecoraro, V.; Xu, G.Y.; Wei, L.; Yu, S.P. Neonatal inflammatory pain and systemic inflammatory responses as possible environmental factors in the development of autism spectrum disorder of juvenile rats. J. Neuroinflammation 2016, 13, 109.
- Iannucci, A.; Caneparo, V.; Raviola, S.; Debernardi, I.; Colangelo, D.; Miggiano, R.; Griffante, G.; Landolfo, S.; Gariglio, M.; De Andrea, M. Toll-like receptor 4-mediated inflammation triggered by extracellular IFI16 is enhanced by lipopolysaccharide binding. PLoS Pathog. 2020, 16, e1008811.
- Alabdali, A.; Al-Ayadhi, L.; El-Ansary, A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflammation 2014, 11, 4.
- Geschwind, D.H.; Levitt, P. Autism spectrum disorders: Developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111.
- Long, Z.; Duan, X.; Mantini, D.; Chen, H. Alteration of functional connectivity in autism spectrum disorder: Effect of age and anatomical distance. Sci. Rep. 2016, 6, 26527.
- Goh, K.K.; Chen, C.Y.; Wu, T.H.; Chen, C.H.; Lu, M.L. Crosstalk between Schizophrenia and Metabolic Syndrome: The Role of Oxytocinergic Dysfunction. Int. J. Mol. Sci. 2022, 23, 7092.
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17.
- Montag, C.; Brockmann, E.M.; Bayerl, M.; Rujescu, D.; Müller, D.J.; Gallinat, J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: A case-control study. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2013, 14, 500–508.
- Goh, K.K.; Chen, C.H.; Lane, H.Y. Oxytocin in Schizophrenia: Pathophysiology and Implications for Future Treatment. Int. J. Mol. Sci. 2021, 22, 2146.
- Young, J.W.; Geyer, M.A. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J. Psychopharmacol. 2015, 29, 178–196.
- Millan, M.J.; Bales, K.L. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: The CNTRICS initiative. Neurosci. Biobehav. Rev. 2013, 37, 2166–2180.
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288.
- Ferguson, J.N.; Aldag, J.M.; Insel, T.R.; Young, L.J. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 8278–8285.
- Crawley, J.N.; Chen, T.; Puri, A.; Washburn, R.; Sullivan, T.L.; Hill, J.M.; Young, N.B.; Nadler, J.J.; Moy, S.S.; Young, L.J.; et al. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides 2007, 41, 145–163.
- Popik, P.; van Ree, J.M. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur. Neuropsychopharmacol. 1991, 1, 555–560.
- Kirsch, P.; Esslinger, C.; Chen, Q.; Mier, D.; Lis, S.; Siddhanti, S.; Gruppe, H.; Mattay, V.S.; Gallhofer, B.; Meyer-Lindenberg, A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 11489–11493.




