Early-life stress during critical periods of brain development can have long-term effects on physical and mental health. Oxytocin is a critical social regulator and anti-inflammatory hormone that modulates stress-related functions and social behaviors and alleviates diseases. Oxytocin-related neural systems show high plasticity in early postpartum and adolescent periods. Early-life stress can influence the oxytocin system long term by altering the expression and signaling of oxytocin receptors. Deficits in social behavior, emotional control, and stress responses may result, thus increasing the risk of anxiety, depression, and other stress-related neuropsychiatric diseases. Oxytocin is regarded as an important target for the treatment of stress-related neuropsychiatric disorders.
- early-life stress
- oxytocin
- neural circuit
- neuropsychiatric disorders
1. Introduction
2. The Properties of Oxytocin
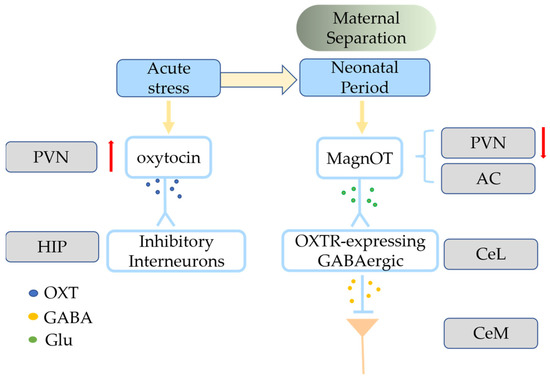
Oxytocin is a neuropeptide hormone primarily synthesized in the brain by the parvocellular neurons (parvOT) of the paraventricular nucleus (PVN) and the magnocellular neurons (magnOT) of both the PVN and supraoptic nucleus (SON) [11][12][13][14][15,16,17,18]. The magnOT neurons primarily innervate the forebrain and release oxytocin through the posterior pituitary gland into the bloodstream to supply the body. The parvOT neurons release less oxytocin, mainly to brainstem nuclei, the spinal cord and amygdala, stria bed nucleus, nucleus accumbens (NAc), magnOT neurons of the SON, and other regions [15][16][17][19,20,21]. The oxytocin gene encodes the structural precursor to oxytocin and is expressed in the mammalian hypothalamus; oxytocin is stored in large, dense-core vesicles [18][19][22,23]. The axonal projections of PVN and SON pass through the median eminence and innervate the posterior pituitary lobe, thereby releasing OXT into circulation or through volume transfer to nerve tissue to regulate physiology [20][24]. Oxytocin works through its receptor, OXTR [21][22][29,30], which belongs to the GPCR family and can activate several different G-protein-induced signaling cascades [23][24][31,32]. OXTR activation has been associated with two separate intracellular signaling cascades with dependence on either: Gαi/o or Gαq [24][25][28,32]. OXTR-expressing neurons have different functionality in different brain regions: social reward in the ventral tegmental area (VTA) [26][33], social recognition in the anterior olfactory nucleus (AON) [27][34], and social memory in the hippocampal CA2 region [28][35]. OXTRs are distributed in neurons, astrocytes, and microglia [24][29][32,36]. Neuronal oxytocin signaling is primarily influenced by the amount of locally released oxytocin, OXTR affinity, density, local enzymatic cleavage, and the resulting concentration of oxytocin in the extracellular fluid [25][28]. Oxytocin treatment reduces inflammation and the severity of various diseases. OXTRs have been found on immune cells, including neutrophils, macrophages, and lymphocytes [30][47]. During inflammation, nuclear factor kappa-light-chain-enhancer (NF-κB) mediates increased OXTR expression in macrophages [31][48]. Oxytocin can inhibit the macrophage transition to active inflammatory cells by promoting the expression of β-arrestin 2 [30][47] and peroxisome proliferator-activated receptor gamma [32][33][49,50].3. Oxytocin-Involved Social Behavior
3.1. Positive Social Interactions
Oxytocin signaling is mediated by release from afferent terminals to receptors present in various target regions that impact aspects of behavior. Oxytocin modulates a variety of positive social behaviors via OXTRs in many limbic and reward-related regions of the mammalian brain, such as the PFC, NAc, amygdala, lateral septum, and thalamus [34][54]. The VTA and the NAc of the limbic system of the midbrain cortex are abundant in OXTRs and are linked to dopaminergic reward motivation [26][33]. The VTA PVN neurons release oxytocin to dopamine-secreting neurons of the nerve bundles that stretch from the VTA to the NAc, thus strengthening the social abilities of these mice [26][35][33,55]. Oxytocin may also increase the excitatory drive of VTA dopamine neurons that project to the NAc, thus improving sociability and social pleasure and increasing 5-HT release in the NAc [26][36][37][38][33,56,57,58]. The potent monoamine releaser (±) 3,4-methylenedioxymethamphetamine (MDMA) has potent prosocial effects in humans. Beyond social reward behavior, oxytocin also regulates prosocial behavior and the parent-child relationship. Countless studies have shown that oxytocin signaling regulates social interactions, especially prosocial behaviors associated with altruism [39][60]. MagnOT neurons have long-distance axonal projections to forebrain regions, including the PFC, AON, NAc, lateral septum, hippocampus, and medial and central amygdala, which are found only in higher vertebrates such as mammals and reptiles. These co-evolved regions are implicated in complex social and emotional behaviors [25][28]. In social mammals, OXT mediates prosocial behaviors such as mate preference, social approval and proximity, and parental care, and this effect is more pronounced in monogamous mating systems [40][61]. Social monogamy is a mating system characterized by sharing of territory among partners, mutual care, and preferential mating [41][62]. Neurobiological research on animal social bonds has focused on “social monogamous” species because they have strong long-term bonds, and the formation of this social bond seems to involve oxytocin [42][63]. The density of OXTR is higher in the NAc, mPFC, and amygdala of monogamous meadow voles than in non-monogamous montane (Microtus montanus) and meadow (Microtus pennsylvanicus) voles [41][43][62,64]. OXTR mRNA expression is present in the NAc of humans but absent from non-monogamous rhesus monkeys [41][44][45][62,65,66], and oxytocin is activated in the ACC and amygdala when rats engage in helping behaviors [46][67]. Oxytocin has a crucial role in complex social behaviors such as generosity, empathy, and collaboration [47][78]. Wild-type zebrafish freeze when observing a conspecific in pain in an isolated tank, whereas mutant zebrafish lineages lacking OXT or OXTR or do not exhibit these fear responses, indicating that oxytocin is necessary and sufficient for social fear contagion in zebrafish [48][79]. Interest in the role of oxytocin in social cognition and emotional processing increased after a study found that an oxytocin nasal spray promotes trust and play in social situations [49][80]. Complex social behaviors are closely connected with analgesia, and the role of oxytocin in analgesia and fear is noteworthy. The lactation-induced and oxytocin-dependent lack of social dread is blocked by chemogenetic suppression of DREADD-expressing OXT+ neurons that project to the lateral septum in breastfeeding mice [50][81]. The freezing caused by conventional fear training is reduced by oxytocin in the central amygdala (instead of a maternal context) [17][21]. The lateral portion of the central amygdala (CeL) receives lateral branch axons from magnOT neurons in the PVN and accessory nuclei, and these neurons create glutamatergic synapses with OXTR-expressing GABAergic neurons (Figure 12) [17][21].