
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | 俊达 刘 | -- | 2128 | 2023-08-14 11:56:16 | | | |
| 2 | 俊达 刘 | + 1253 word(s) | 3381 | 2023-08-18 11:56:23 | | | | |
| 3 | 俊达 刘 | Meta information modification | 3381 | 2023-08-18 12:00:25 | | | | |
| 4 | 俊达 刘 | Meta information modification | 3381 | 2023-08-18 12:00:47 | | | | |
| 5 | 俊达 刘 | Meta information modification | 3381 | 2023-08-18 12:02:11 | | |
Video Upload Options
Pressure hydrometallurgy has attracted much attention for its characteristics, such as the high adaptability of raw materials and environmental friendliness. Flashing (flash boiling or flash evaporation) refers to the phase change phenomenon from liquid to gas triggered by depressurization, which is an important connection between high-pressure processes and atmospheric ones in pressure hydrometallurgy.
1. Introduction
Hydrometallurgy is a technology of separating, enriching, and extracting metals in which the valuable metal components of ores, calcine, and other materials are dissolved or precipitated in the solution by a leaching agent. As the concentration of mineable materials declines and the guidelines for environmental protection increase, hydrometallurgy technology at atmospheric pressure struggles to satisfy the demand for the extraction of non-ferrous metals from complex minerals and the comprehensive utilization of rare metals. Therefore, pressure hydrometallurgy has been rapidly developed and has become the most important modern hydrometallurgy technology.
As the name suggests, pressure hydrometallurgy is carried out in higher-pressure conditions. In essence, the reaction temperature of an aqueous solution can be much higher than its boiling point in the atmosphere if the operational pressure increases, e.g., 200~300 °C. This will greatly enhance the reaction driving force and increase the chemical reaction rate in the metallurgical process. Compared with traditional hydrometallurgy, pressure hydrometallurgy technology has the following characteristics [1]:
- A good adaptability of raw materials and a high comprehensive utilization of resources. In the leaching kinetic conditions of high temperature and pressure, the pressure hydrometallurgy process has a fast leaching rate and is able to treat a variety of metal sulfide and oxide ores (see Figure 1) as well as complex low-grade materials, including low-grade minerals[2], arsenic-containing materials[3], multi-metal-accompanying minerals, intermediate metallurgical materials, and secondary renewable resources. While extracting the main metal, it can also selectively leach and separate the accompanying rare metals or precious metals[4]. Consequently, it helps to improve the comprehensive recovery of the accompanying elements whose economic benefit even exceeds that of the main metal. Therefore, pressure hydrometallurgy technology has been widely used in the fields of extraction metallurgy and materials preparation for aluminum, uranium, copper, zinc, nickel, cobalt, tungsten, and a variety of rare and precious metals.
- A short technological process and environmental friendliness. Pressure hydrometallurgy can directly leach sulfide ores and convert the sulfur element in the ore into sulfur monomers. In this way, the processes of oxidation roasting and sulfuric acid production are not needed. Compared with traditional hydrometallurgy, pressure hydrometallurgy significantly shortens the production process and avoids releasing SO2 pollution into the air. In addition, the sulfur monomers from pressure hydrometallurgy are easier to store and transport than the sulfuric acid from traditional hydrometallurgy.
High cost and difficult operation. Compared to traditional hydrometallurgy, a larger cost is necessary to build the production line for pressure hydrometallurgy because the high-temperature and high-pressure reactors and their ancillary equipment are always very expensive. Meanwhile, it is difficult to operate the high-temperature and high-pressure equipment smoothly, which results in technicians with a high level of operation and management being needed.
Despite the advantages of pressure hydrometallurgy, it does come with significant challenges. Among these is the management of the leaching process, which is a key aspect of pressure hydrometallurgy and has become a major topic in the field, such as catalysts for the leaching process[5], leaching medium[6], dispersants[7], etc. Additionally, the phenomenon of flash boiling presents potential safety risks that must be carefully managed.
2. Flashing Phenomena in the Oxygen Pressure Leaching Process of Zinc Sulfide
Concentrate
2.1. The Oxygen Pressure Leaching Technology of Zinc Sulfide Concentrate
In the oxygen pressure direct leaching process of zinc sulfide concentrate, the finely ground concentrate with a particle size of 40~60 μm is mixed with waste electrolyte and a sulfuric acid solution. The mixture, called a slurry, is pumped into an autoclave by diaphragm pumps, where acid leaching is conducted. The oxygen is injected into the autoclave to promote the conversion of zinc sulfide into zinc sulfate and sulfur monomers. The main chemical reaction is as follows:
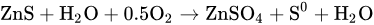
The higher the slurry temperature, the faster the leaching reaction rate[8]. But, when the temperature exceeds the melting point of sulfur, the product sulfur will be melted and cover the unreacted sulfide. This phenomenon will hinder the leaching reaction, which is called a passivation reaction. The following measures can be applied to avoid the passivation reaction:
- The leaching slurry temperature is maintained below the melting point of sulfur monomers (about 120 °C) by adjusting the steam flux of the autoclave.
- When the leaching slurry temperature is demanded to be over the melting point of sulfur monomers, some additives, such as lignosulfonates[9], are used to disperse the sulfur covering the unreacted sulfide.
The two-stage oxygen pressure leaching technology of zinc sulfide concentrates is widely used in China. Its flowchart is shown in Figure 2.
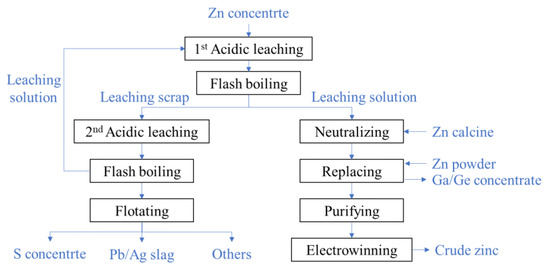
Figure 2. The flow chart of two-stage oxygen pressure leaching process of zinc sulfide concentrate.
In the first acidic leaching, the slurry is made from the ground zinc concentrate mixed with the waste acid and the solution of the second leaching and then pumped into the autoclave. About 50% zinc is leached from the minerals after the slurry stay 1.5~2 h in the autoclave at a temperature of 105~115 °C. The leached slurry with a high temperature and high pressure is depressurized into the atmosphere state in the flash boiling devices. By filtrating the slurry, the leaching solution and scrap are separated. The solution is rich in gallium, germanium, zinc, and other metal ions as well as residual sulfuric acid. So, zinc oxide is firstly added to neutralize the residual sulfuric acid, then zinc powder is added to replace gallium, germanium, and other metal ions. By precipitating and filtrating the zinc sulfate solution, the gallium–germanium enrichments[11] are extracted. The zinc sulfate solution is purified by iron removal and then the crude zinc is produced by electrowinning procedure.
About 50% zinc element is left in the leaching scrap of the first acidic leaching, and the scrap is needed to be further leached in the second acidic leaching. The low grade of zinc and high content of sulfur monomers make the leaching dynamic condition poorer than that of the first stage. To improve the leaching rate, the second acidic leaching is conducted in the case of a high acid concentration (80~85 g/L) and high temperature (150~155 °C), and the leaching time extends to 2~4 h. The slurry is also depressurized through the flash boiling devices. The sulfur and Pb/Ag slag are orderly separated from the scrap of the second leaching by flotation. The solution of the second leaching contains a high concentration of acid and is returned to the first acidic leaching.
2.2. The Flashing of Zinc Sulfide Leaching Slurry
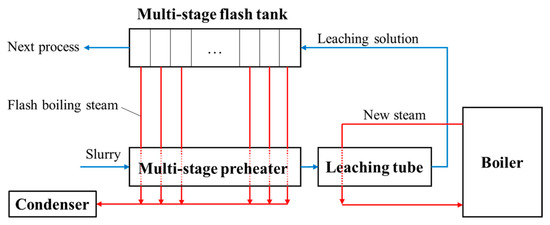
As shown in Figure 2, each leaching procedure connects to a flash boiling procedure in the two-stage oxygen pressure leaching process of zinc sulfide concentrate. The flash boiling occurs when the leaching slurry flows into the flash tank whose pressure is lower than the saturation pressure of the slurry. The boiling rate is controlled by adjusting the pressure of the discharge steam pipeline to regulate the superheat degree of the slurry. In the flash boiling process, a large amount of steam is generated and takes away heat energy from the slurry, which makes the slurry temperature and pressure decrease rapidly until saturation is achieved.
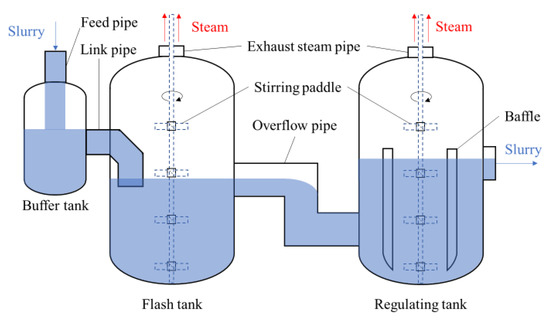
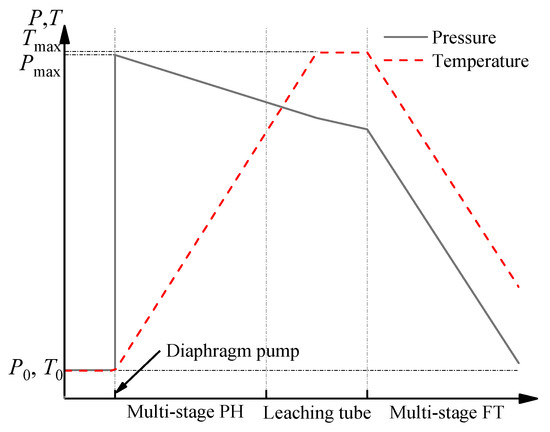
The same flashing devices are configured for the first and second leaching stage, including the flash tank (FT), buffer tank, and regulating tank (RT) (seeing Figure 3). Their different operation parameters are shown in Table 2. It is known that the temperature and pressure of the slurry in the second leaching are higher than those in the first leaching. Correspondingly, the temperature of FT for the second leaching is higher than that of FT for the first leaching.
3. Flashing Phenomena in BAYER Method for Alumina Production
3.1. The Alumina Production Technology in Bayer Method
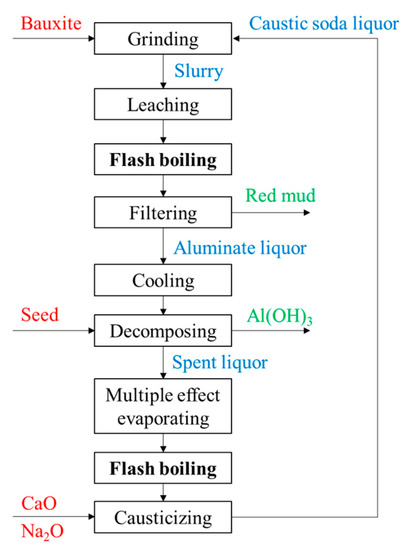
3.2. The Flashing of Alumina Leaching Slurry
4. Characteristics of Flashing in Pressure Hydrometallurgy
5. Flashing in Pressure Hydrometallurgy
- (a)
-
Process simulation: Hydrometallurgical processes have been embedded in many commercial process simulation software, such as ASPEN [23] and HSC Sim [24]. The performance of a flashing system and the impact of the process parameters on its production efficiency can be studied and analyzed using the above software.
- (b)
-
Thermal analysis and energy conservation of the spent liquor evaporation process: As mentioned above, the evaporation process of spent liquor is an important process in the alumina production process. However, the flashing of spent liquor in an alumina refinery has the disadvantages of high steam consumption per ton, severe heat exchanger tube scarring, and low evaporation capacity [26].
- (c)
-
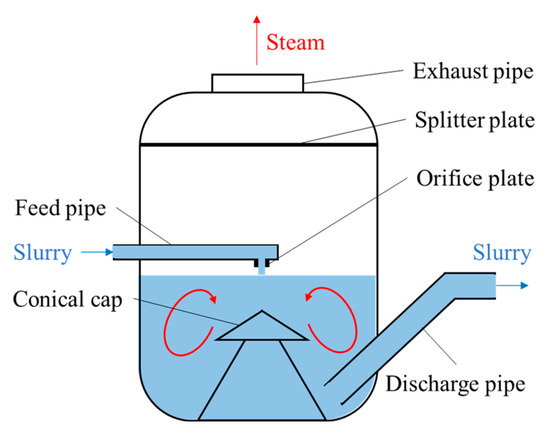
The optimized design for the feed orifice plate of an alumina slurry flash tank: As shown in Figure 7, the alumina slurry flash tank is generally a large cylinder. Its bottom half contains the slurry, while its top half is used for separating the flash steam from the slurry and discharging the steam from the top of the tank. The slurry with a high temperature and high pressure is sprayed into the flash tank through a vertical downward orifice plate located at the end of the feed pipe. As mentioned earlier, many efforts in optimizing the flash tank have been made to prolong its life and reduce production costs. Most of them focused on the design of the feed orifice plate. A conventional feed orifice plate consists of a plug valve and a section of equal cross-section plunger [27,28] where most of the pressure drop occurs. This feed orifice plate is prone to generating explosive flashing when the volume expansion rate of the slurry is high (e.g., at low pressure), which will cause severe wear on the flash tank wall and on the components near the orifice plate. Williams [29] suggested that an expansion cone be added below the plunger, and adjusting the diameter of the plunger and the expansion cone exit can decrease the pressure drop between the upstream of orifice plate and inside of flash tank, which hence weakens the shock wave intensity of the slurry. Smith et al. [27,28] called Williams’ design a “rocket nozzle” and pointed out that it does prevent explosive flashing and minimize the wear and tear of the pipe from explosive flashing, but the jetting flow can cause severe wear on the bottom of the groove or on the conical cap (see Figure 7). They designed a “flashtube” (Figure 9), whose opening cross-sectional area is proportional to the linear position of the plug with a parabolic profile. By adjusting the plug position to expand the slurry to a low enough pressure in the flashtube, the shock wave will be formed inside the flashtube rather than in the receiving tank. When shock is formed and the resistance loss increases, the velocity and kinetic energy of the slurry at the outlet of the flashtube drop, thus reducing the impact wear on the bottom and the conical cap of the flash tank.
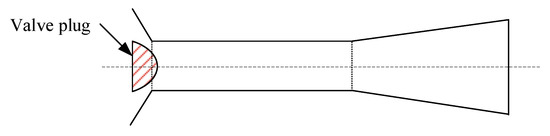 Figure 9. Schematic geometry of the flashtube. Reprinted with permission from Ref. [27]. 2005, Elsevier.
Figure 9. Schematic geometry of the flashtube. Reprinted with permission from Ref. [27]. 2005, Elsevier. - (d)
-
Numerical simulation of multi-phase flow and heat transfer process: Numerical simulations of the flashing process in pressure hydrometallurgy are scarce and still in the early stages of development. Smith et al. [27] performed a simple one-dimensional numerical analysis for their design of a flashtube feed nozzle. The slurry flow in the flashtube was assumed to be in adiabatic and interphase thermal equilibrium, i.e., no heat exchange between the liquid, vapor, and solid particles. These assumptions are acceptable in the case of a large number of solid particles uniformly dispersed within the slurry. Since these particles could provide enough bubble nucleation sites to allow the liquid to vaporize rapidly after the saturation temperature is exceeded, which shortens the metastable state time of the superheated liquid. In addition, they assumed that there is no relative slip between the vapor, liquid, and solid phases, and that the three phases flow at the same velocity throughout the system, which is difficult to satisfy when there are large solid particles or bubbles in the system. Also, the authors pointed out that it is difficult to obtain reliable data to verify the model due to the harsh environment in which flashtubes actually operate, but such simplified models are generally recognized as useful basic design tools in engineering fields.




