Pressure hydrometallurgy has attracted much attention for its characteristics, such as the high adaptability of raw materials and environmental friendliness. Flashing (flash boiling or flash evaporation) refers to the phase change phenomenon from liquid to gas triggered by depressurization, which is an important connection between high-pressure processes and atmospheric ones in pressure hydrometallurgy.压力湿法冶金因其原材料适应性强、环境友好等特点而备受关注。闪蒸(闪蒸或闪蒸)是指减压引发的由液体到气体的相变现象,是压力湿法冶金中高压过程与常压过程的重要联系。
- flashing process
- flash tank
- numerical simulation
- pressure hydrometallurgy
1. Introduction 简介
Hydrometallurgy is a technology of separating, enriching, and extracting metals in which the valuable metal components of ores, calcine, and other materials are dissolved or precipitated in the solution by a leaching agent. As the concentration of mineable materials declines and the guidelines for environmental protection increase, hydrometallurgy technology at atmospheric pressure struggles to satisfy the demand for the extraction of non-ferrous metals from complex minerals and the comprehensive utilization of rare metals. Therefore, pressure hydrometallurgy has been rapidly developed and has become the most important modern hydrometallurgy technology.
- (1)
-
原料适应性好,资源综合利用率高。在高温高压的浸出动力学条件下,压力湿法冶金工艺浸出速度快,能够处理多种金属硫化物和氧化物矿石以及复杂的低品位材料,包括低品位矿物[2],含砷材料[3],多金属伴生矿物,中间冶金材料和二次再生资源。在提取主要金属的同时,还可以选择性地浸出和分离伴随的稀有金属或贵金属[4]。因此,它有助于提高伴随元素的综合回收率,其经济效益甚至超过主要金属。因此,压力湿法冶金技术已广泛应用于铝、铀、铜、锌、镍、钴、钨以及各种稀有贵金属的提取冶金和材料制备领域。
- (2)
-
工艺流程短,环境友好。压力湿法冶金可以直接浸出硫化物矿石,将矿石中的硫元素转化为硫单体。这样,就不需要氧化焙烧和硫酸生产的过程。与传统的湿法冶金相比,压力湿法冶金显著缩短了生产工艺,避免了SO2污染到空气中。此外,来自压力湿法冶金的硫单体比传统湿法冶金的硫酸更容易储存和运输。
As the name suggests, pressure hydrometallurgy is carried out in higher-pressure conditions. In essence, the reaction temperature of an aqueous solution can be much higher than its boiling point in the atmosphere if the operational pressure increases, e.g., 200~300 °C. This will greatly enhance the reaction driving force and increase the chemical reaction rate in the metallurgical process. Compared with traditional hydrometallurgy, pressure hydrometallurgy technology has the following characteristics [1]:
- A good adaptability of raw materials and a high comprehensive utilization of resources. In the leaching kinetic conditions of high temperature and pressure, the pressure hydrometallurgy process has a fast leaching rate and is able to treat a variety of metal sulfide and oxide ores (see Figure 1) as well as complex low-grade materials, including low-grade minerals[2], arsenic-containing materials[3], multi-metal-accompanying minerals, intermediate metallurgical materials, and secondary renewable resources. While extracting the main metal, it can also selectively leach and separate the accompanying rare metals or precious metals[4]. Consequently, it helps to improve the comprehensive recovery of the accompanying elements whose economic benefit even exceeds that of the main metal. Therefore, pressure hydrometallurgy technology has been widely used in the fields of extraction metallurgy and materials preparation for aluminum, uranium, copper, zinc, nickel, cobalt, tungsten, and a variety of rare and precious metals.
- A short technological process and environmental friendliness. Pressure hydrometallurgy can directly leach sulfide ores and convert the sulfur element in the ore into sulfur monomers. In this way, the processes of oxidation roasting and sulfuric acid production are not needed. Compared with traditional hydrometallurgy, pressure hydrometallurgy significantly shortens the production process and avoids releasing SO2 pollution into the air. In addition, the sulfur monomers from pressure hydrometallurgy are easier to store and transport than the sulfuric acid from traditional hydrometallurgy.
High cost and difficult operation. Compared to traditional hydrometallurgy, a larger cost is necessary to build the production line for pressure hydrometallurgy because the high-temperature and high-pressure reactors and their ancillary equipment are always very expensive. Meanwhile, it is difficult to operate the high-temperature and high-pressure equipment smoothly, which results in technicians with a high level of operation and management being needed.
Despite the advantages of pressure hydrometallurgy, it does come with significant challenges. Among these is the management of the leaching process, which is a key aspect of pressure hydrometallurgy and has become a major topic in the field, such as catalysts for the leaching process[
尽管压力湿法冶金具有优势,但它确实带来了重大挑战。其中包括浸出过程的管理,这是压力湿法冶金的一个关键方面,已成为该领域的一个主要主题,例如浸出过程的催化剂[5], leaching medium[
],浸出介质[6], dispersants[
],分散剂[7], etc. Additionally, the phenomenon of flash boiling presents potential safety risks that must be carefully managed.
]等。此外,闪蒸沸现象存在潜在的安全风险,必须谨慎管理。2. Flashing Phenomena in the Oxygen Pressure Leaching Process of Zinc Sulfide
Concentrate二、硫化锌精矿氧压浸出过程中的闪蒸现象
2.1. The Oxygen Pressure Leaching Technology of Zinc Sulfide Concentrate硫化锌精矿的氧气压力浸出技术
在硫化锌精矿的氧压直接浸出过程中,将粒径为40~60μm的细磨精矿与废电解质和硫酸溶液混合。称为浆料的混合物通过隔膜泵泵送入高压釜中,在那里进行酸浸。将氧气注入高压釜中,促进硫化锌转化为硫酸锌和硫单体。主要化学反应如下:In the oxygen pressure direct leaching process of zinc sulfide concentrate, the finely ground concentrate with a particle size of 40~60 μm is mixed with waste electrolyte and a sulfuric acid solution. The mixture, called a slurry, is pumped into an autoclave by diaphragm pumps, where acid leaching is conducted. The oxygen is injected into the autoclave to promote the conversion of zinc sulfide into zinc sulfate and sulfur monomers. The main chemical reaction is as follows:
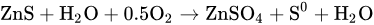
The higher the slurry temperature, the faster the leaching reaction rate[
浆料温度越高,浸出反应速度越快[8]. But, when the temperature exceeds the melting point of sulfur, the product sulfur will be melted and cover the unreacted sulfide. This phenomenon will hinder the leaching reaction, which is called a passivation reaction. The following measures can be applied to avoid the passivation reaction:
]。但是,当温度超过硫的熔点时,产物硫将被熔化并覆盖未反应的硫化物。这种现象会阻碍浸出反应,称为钝化反应。可以采取以下措施来避免钝化反应:- (1)
-
通过调节高压釜的蒸汽通量,将浸出浆料温度保持在硫单体的熔点(约120°C)以下。
- (2)
-
当要求浸出浆料温度超过硫单体的熔点时,使用一些添加剂,如木质素磺酸盐[9],来分散覆盖未反应硫化物的硫。
- The leaching slurry temperature is maintained below the melting point of sulfur monomers (about 120 °C) by adjusting the steam flux of the autoclave.
- When the leaching slurry temperature is demanded to be over the melting point of sulfur monomers, some additives, such as lignosulfonates[9], are used to disperse the sulfur covering the unreacted sulfide.
The two-stage oxygen pressure leaching technology of zinc sulfide concentrates is widely used in China. Its flowchart is shown in Figure
硫化锌精矿的两级氧压浸出技术在我国得到广泛应用。其流程图如图2.
所示。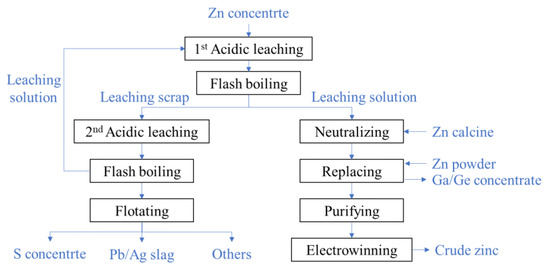
Figure 图2. The flow chart of two-stage oxygen pressure leaching process of zinc sulfide concentrate.
硫化锌精矿两级氧压浸出工艺流程图.In the first acidic leaching, the slurry is made from the ground zinc concentrate mixed with the waste acid and the solution of the second leaching and then pumped into the autoclave. About 50% zinc is leached from the minerals after the slurry stay 1.5~2 h in the autoclave at a temperature of 105~115 °C. The leached slurry with a high temperature and high pressure is depressurized into the atmosphere state in the flash boiling devices. By filtrating the slurry, the leaching solution and scrap are separated. The solution is rich in gallium, germanium, zinc, and other metal ions as well as residual sulfuric acid. So, zinc oxide is firstly added to neutralize the residual sulfuric acid, then zinc powder is added to replace gallium, germanium, and other metal ions. By precipitating and filtrating the zinc sulfate solution, the gallium–germanium enrichments[
在第一次酸性浸出中,将研磨的锌精矿与废酸和溶液混合而成,然后泵入高压釜中。浆料在高压釜中在50~1°C的温度下停留5.2~105 h后,从矿物质中浸出约115%的锌。 浸出的浆料在高温高压下在闪蒸沸腾装置中减压进入大气状态。通过过滤浆料,分离浸出溶液和废料。该溶液富含镓、锗、锌和其他金属离子以及残留的硫酸。因此,首先加入氧化锌以中和残留的硫酸,然后加入锌粉以取代镓,锗和其他金属离子。通过沉淀和过滤硫酸锌溶液,提取镓-锗富集物[11] are extracted. The zinc sulfate solution is purified by iron removal and then the crude zinc is produced by electrowinning procedure.
]。About 50% zinc element is left in the leaching scrap of the first acidic leaching, and the scrap is needed to be further leached in the second acidic leaching. The low grade of zinc and high content of sulfur monomers make the leaching dynamic condition poorer than that of the first stage. To improve the leaching rate, the second acidic leaching is conducted in the case of a high acid concentration (80~85 g/L) and high temperature (150~155 °C), and the leaching time extends to 2~4 h. The slurry is also depressurized through the flash boiling devices. The sulfur and Pb/Ag slag are orderly separated from the scrap of the second leaching by flotation. The solution of the second leaching contains a high concentration of acid and is returned to the first acidic leaching.
第一次酸性浸出的浸出废料中留下约50%的锌元素,需要在第二次酸性浸出中进一步浸出废料。锌品位低,硫单体含量高,使浸出动态条件比第一阶段差。为提高浸出速率,在高酸浓度(80~85 g/L)和高温(150~155 °C)的情况下进行第二次酸性浸出,浸出时间延长至2~4 h。浆料也通过闪蒸煮沸装置减压。硫和Pb/Ag渣通过浮选从第二次浸出的废料中有序分离。第二次浸出的溶液含有高浓度的酸,并返回到第一次酸性浸出。2.2. The Flashing of Zinc Sulfide Leaching Slurry硫化锌浸出浆液的闪蒸
As shown in Figure
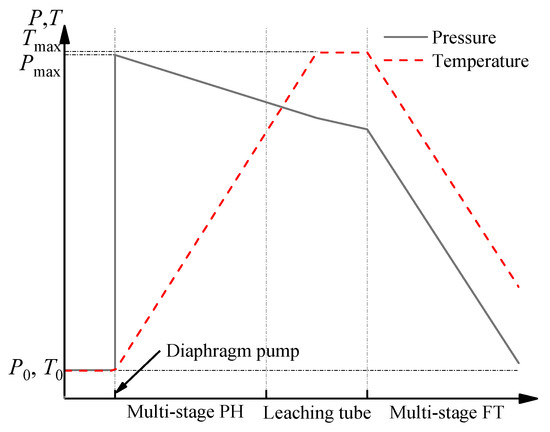
如图2, each leaching procedure connects to a flash boiling procedure in the two-stage oxygen pressure leaching process of zinc sulfide concentrate. The flash boiling occurs when the leaching slurry flows into the flash tank whose pressure is lower than the saturation pressure of the slurry. The boiling rate is controlled by adjusting the pressure of the discharge steam pipeline to regulate the superheat degree of the slurry. In the flash boiling process, a large amount of steam is generated and takes away heat energy from the slurry, which makes the slurry temperature and pressure decrease rapidly until saturation is achieved.
所示,每个浸出程序都连接到硫化锌精矿两级氧气压力浸出过程中的闪蒸煮过程。当浸出浆液流入压力低于浆料饱和压力的闪蒸罐时,就会发生闪蒸沸腾。通过调节排放蒸汽管道的压力来控制沸腾速率,以调节浆料的过热度。在闪蒸过程中,产生大量的蒸汽,带走浆料中的热能,使浆料温度和压力迅速下降,直到达到饱和。The same flashing devices are configured for the first and second leaching stage, including the flash tank (FT), buffer tank, and regulating tank (RT) (seeing Figure
第一和第二浸出阶段配置了相同的闪蒸装置,包括闪蒸罐(FT),缓冲罐和调节罐(RT)(见图3). Their different operation parameters are shown in Table 2. It is known that the temperature and pressure of the slurry in the second leaching are higher than those in the first leaching. Correspondingly, the temperature of FT for the second leaching is higher than that of FT for the first leaching.
)。众所周知,第二次浸出时浆料的温度和压力高于第一次浸出时的温度和压力。相应地,第二次浸出的FT温度高于第一次浸出的FT温度。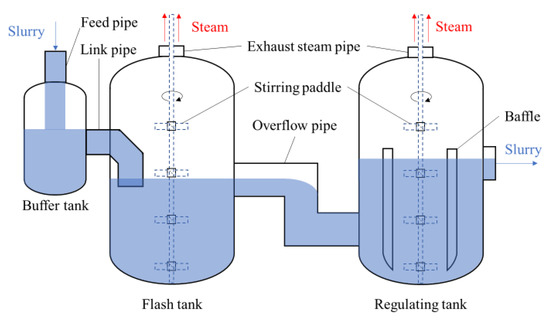 Figure 3. Schematic structure of flash boiling devices.The slurry passes through the buffer tank, flash tank, and regulating tank in turn. The flash tank is the main vessel for the depressurization of the slurry. The buffer tank is first used to pre-reduce the pressure partially, which can avoid the shock wave caused by rapid depressurizing at the outlet of the pipe. The primary function of the regulating tank lies in further depressurization of the slurry and controlling the crystallization process of sulfur.There is only one inlet and one outlet in the buffer tank. The leached slurry flows into the buffer tank via the feed pipe at the top. The mixture of flash steam and the slurry flows into the flash tank. To achieve moderate depressurization, the link pipe of the flash tank is immersed in the liquid. The slurry outflows from the flash tank into the regulating tank through the overflow pipe, which connects the middle of the flash tank and the bottom of the regulating tank.
Figure 3. Schematic structure of flash boiling devices.The slurry passes through the buffer tank, flash tank, and regulating tank in turn. The flash tank is the main vessel for the depressurization of the slurry. The buffer tank is first used to pre-reduce the pressure partially, which can avoid the shock wave caused by rapid depressurizing at the outlet of the pipe. The primary function of the regulating tank lies in further depressurization of the slurry and controlling the crystallization process of sulfur.There is only one inlet and one outlet in the buffer tank. The leached slurry flows into the buffer tank via the feed pipe at the top. The mixture of flash steam and the slurry flows into the flash tank. To achieve moderate depressurization, the link pipe of the flash tank is immersed in the liquid. The slurry outflows from the flash tank into the regulating tank through the overflow pipe, which connects the middle of the flash tank and the bottom of the regulating tank.3. Flashing Phenomena in BAYER Method for Alumina Production
3.1. The Alumina Production Technology in Bayer Method
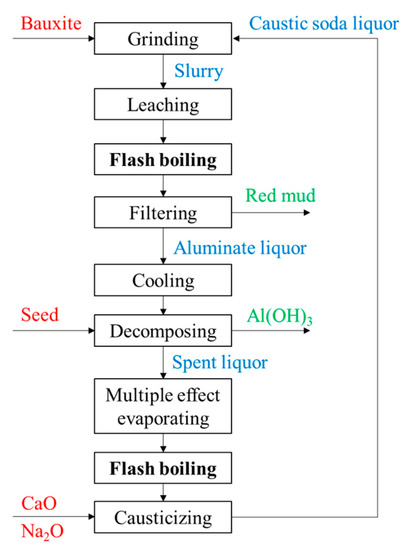
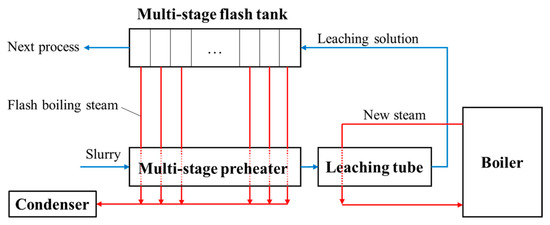
The Bayer method is most widely used in the alumina production process, and the major reactions are as follows: where x = 1 for monohydrate hard alumina bauxite or monohydrate soft alumina bauxite; x = 3 for the trihydrate alumina bauxite. Among them, trihydrate alumina bauxite is the easiest to leach, with a typical leaching temperature of 140~145 °C and Na2O mass concentration of 120~140 g/L. Monohydrate soft alumina bauxite is more difficult to leach, requiring a leaching temperature over 200 °C and a Na2O mass concentration of 180~240 g/L. Monohydrate hard bauxite is the most difficult to leach, with a leaching temperature of 240~270 °C and a Na2O mass concentration of 240~300 g/L. The main process flow of alumina in the Bayer method is shown in Figure 4. The bauxite is ground and mixed with caustic soda liquor. Their mixture is called a slurry, which is pumped into the leaching device (a leaching tube is generally used) by a diaphragm pump. The slurry is first heated to a high temperature where the alumina is subsequently leached into the solution. Then, the high temperature and pressure mixture is cooled down and depressurized by a multi-stage flash boiling device. Through filtration, the flash-boiled slurry is separated into the insoluble material (namely red mud) and the liquid (namely aluminate liquor). After cooling down, the aluminate liquor becomes supersaturated and starts decomposing.
3.2. The Flashing of Alumina Leaching Slurry
4. Characteristics of Flashing in Pressure Hydrometallurgy
5. Flashing in Pressure Hydrometallurgy
The multi-component three-phase flashing process in pressure hydrometallurgy is very complex, and few studies focus directly on it. On the Web of Science, up until 2023, there have been 3745 search records using the keyword of hydrometallurgy, while there have only been 15 search records using the keywords of hydrometallurgy and flashing, in which almost no literature has directly studied the physical phenomena of the flashing process. The relevant studies fall into the following categories:- (a)
-
Process simulation: Hydrometallurgical processes have been embedded in many commercial process simulation software, such as ASPEN [23] and HSC Sim [24]. The performance of a flashing system and the impact of the process parameters on its production efficiency can be studied and analyzed using the above software.
- (b)
-
Thermal analysis and energy conservation of the spent liquor evaporation process: As mentioned above, the evaporation process of spent liquor is an important process in the alumina production process. However, the flashing of spent liquor in an alumina refinery has the disadvantages of high steam consumption per ton, severe heat exchanger tube scarring, and low evaporation capacity [26].
- (c)
-
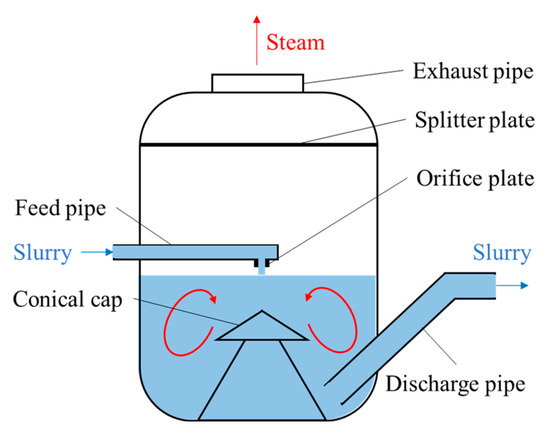
The optimized design for the feed orifice plate of an alumina slurry flash tank: As shown in Figure 7, the alumina slurry flash tank is generally a large cylinder. Its bottom half contains the slurry, while its top half is used for separating the flash steam from the slurry and discharging the steam from the top of the tank. The slurry with a high temperature and high pressure is sprayed into the flash tank through a vertical downward orifice plate located at the end of the feed pipe. As mentioned earlier, many efforts in optimizing the flash tank have been made to prolong its life and reduce production costs. Most of them focused on the design of the feed orifice plate. A conventional feed orifice plate consists of a plug valve and a section of equal cross-section plunger [27,28] where most of the pressure drop occurs. This feed orifice plate is prone to generating explosive flashing when the volume expansion rate of the slurry is high (e.g., at low pressure), which will cause severe wear on the flash tank wall and on the components near the orifice plate. Williams [29] suggested that an expansion cone be added below the plunger, and adjusting the diameter of the plunger and the expansion cone exit can decrease the pressure drop between the upstream of orifice plate and inside of flash tank, which hence weakens the shock wave intensity of the slurry. Smith et al. [27,28] called Williams’ design a “rocket nozzle” and pointed out that it does prevent explosive flashing and minimize the wear and tear of the pipe from explosive flashing, but the jetting flow can cause severe wear on the bottom of the groove or on the conical cap (see Figure 7). They designed a “flashtube” (Figure 9), whose opening cross-sectional area is proportional to the linear position of the plug with a parabolic profile. By adjusting the plug position to expand the slurry to a low enough pressure in the flashtube, the shock wave will be formed inside the flashtube rather than in the receiving tank. When shock is formed and the resistance loss increases, the velocity and kinetic energy of the slurry at the outlet of the flashtube drop, thus reducing the impact wear on the bottom and the conical cap of the flash tank.
-
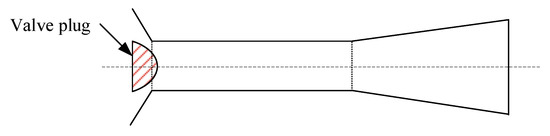 Figure 9. Schematic geometry of the flashtube. Reprinted with permission from Ref. [27]. 2005, Elsevier.
Figure 9. Schematic geometry of the flashtube. Reprinted with permission from Ref. [27]. 2005, Elsevier.- (d)
-
Numerical simulation of multi-phase flow and heat transfer process: Numerical simulations of the flashing process in pressure hydrometallurgy are scarce and still in the early stages of development. Smith et al. [27] performed a simple one-dimensional numerical analysis for their design of a flashtube feed nozzle. The slurry flow in the flashtube was assumed to be in adiabatic and interphase thermal equilibrium, i.e., no heat exchange between the liquid, vapor, and solid particles. These assumptions are acceptable in the case of a large number of solid particles uniformly dispersed within the slurry. Since these particles could provide enough bubble nucleation sites to allow the liquid to vaporize rapidly after the saturation temperature is exceeded, which shortens the metastable state time of the superheated liquid. In addition, they assumed that there is no relative slip between the vapor, liquid, and solid phases, and that the three phases flow at the same velocity throughout the system, which is difficult to satisfy when there are large solid particles or bubbles in the system. Also, the authors pointed out that it is difficult to obtain reliable data to verify the model due to the harsh environment in which flashtubes actually operate, but such simplified models are generally recognized as useful basic design tools in engineering fields.
67. Conclusions
In pressure hydrometallurgy, the flashing process is mainly applied to connect the high-pressure leaching equipment with atmospheric pressure equipment, as well as for evaporation and concentration of the solution. It plays an important role in the smooth running of the metallurgical process, lowering the steam consumption and balancing the liquid of the metallurgical system. A better understanding of the flashing process may contribute to optimizing the design, enhancing the device’s lifetime, and reducing operational costs. The flashing process in pressure hydrometallurgy can be divided into slurry and solution flashing according to the solid holdup in the fluid. After the leaching process of concentrate at high pressure and high temperatures, slurry flashing is used to reduce its pressure and recover its waste heat, while solution flashing is used for the evaporation and concentration of the solution. The combination of multi-effect evaporation and multi-stage flashing can enhance the concentration efficiency. BPE and NEA exist in the flashing process of both slurry and solution, and accurate BPE and NEA are helpful for guiding reasonable multi-stage flashing design to achieve energy conservation and emission reduction. There are hardly any studies on flash evaporation in pressure hydrometallurgy with a few exceptions based on extremely simplified models, such as the one-dimensional homogeneous phase equilibrium model. CFD is a promising tool for the analysis of complex multiphase problems, and considerable progress has been made in other fields.
-
