Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ASHEESH KUMAR | -- | 1850 | 2023-08-14 09:32:05 | | | |
| 2 | Fanny Huang | Meta information modification | 1850 | 2023-08-15 05:09:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rath, G.K.; Pandey, G.; Singh, S.; Molokitina, N.; Kumar, A.; Joshi, S.; Chauhan, G. Carbon Dioxide Separation Technologies. Encyclopedia. Available online: https://encyclopedia.pub/entry/48031 (accessed on 07 February 2026).
Rath GK, Pandey G, Singh S, Molokitina N, Kumar A, Joshi S, et al. Carbon Dioxide Separation Technologies. Encyclopedia. Available at: https://encyclopedia.pub/entry/48031. Accessed February 07, 2026.
Rath, Gourav Kumar, Gaurav Pandey, Sakshi Singh, Nadezhda Molokitina, Asheesh Kumar, Sanket Joshi, Geetanjali Chauhan. "Carbon Dioxide Separation Technologies" Encyclopedia, https://encyclopedia.pub/entry/48031 (accessed February 07, 2026).
Rath, G.K., Pandey, G., Singh, S., Molokitina, N., Kumar, A., Joshi, S., & Chauhan, G. (2023, August 14). Carbon Dioxide Separation Technologies. In Encyclopedia. https://encyclopedia.pub/entry/48031
Rath, Gourav Kumar, et al. "Carbon Dioxide Separation Technologies." Encyclopedia. Web. 14 August, 2023.
Copy Citation
Carbon dioxide (CO2) emissions from burning fossil fuels play a crucial role in global warming/climate change. The effective removal of CO2 from the point sources or atmosphere (CO2 capture), its conversion to value-added products (CO2 utilization), and long-term geological storage, or CO2 sequestration, has captured the attention of several researchers and policymakers.
CCUS
carbon dioxide
Gas hydrates
Net-zero
CO2 Separation
1. Introduction
Energy is the primary source of greenhouse-gas (GHG) emissions, with a share of around 76% (mainly CO2 emissions). Though COVID-19 triggered an exceptional decrease in global GHG emissions in 2020, the largest-ever annual rise in CO2 emissions witnessed a CO2 rise from 31.5 Gt to 36.6 Gt in 2021 [1]. To achieve the COP26 targets established for net zero, carbon-capture utilization and storage (CCUS) technologies could be the bottleneck. CCUS is predominantly employed to capture CO2 produced from different industrial sources, such as steel plants, power plants, chemical industries, and thermal-electric power plants. The most conventional approaches for carbon capture are precombustion, postcombustion, and oxyfuel combustion methods [2]. Although there has been extensive research in direct air-capture approaches, the high capital cost has been a significant challenge to deploying this technique. In addition, the development of CO2 separation techniques (to separate CO2 from flue or fuel–gas mixtures) has also gained a significant attraction for the economical deployment of carbon-capture technologies. In this direction, adsorption, absorption, microbial, membrane separation, and environmentally friendly techniques such as ‘gas hydrate-based’ separation and biological processes have grown significantly around the world [2][3][4]. The adsorption, absorption, and cryogenic distillation processes are the most mature CO2 separation methods with high separation efficiency. CO2 capture through Gas hydrate-based and membrane separation methods is highly effective due to their low energy consumption. However, these technologies also have drawbacks, mainly temperature requirements, energy intensity, and CO2 concentration dependency [3][5][6][7][8][9]. The industrial applications of CO2 capture through different methods have numerous drawbacks, restricting this process from being used commercially.
2. CO2 Separation/Capture Technologies
Research and technological advancements have created multiple novel carbon dioxide (CO2) separation methods. The uncontrollable release of notorious anthropogenic GHGs, especially carbon dioxide, has caused major environmental issues. The harmful effect of CO2 has motivated the development of technologies dedicated to achieving net-zero emission goals while evaluating their efficacy, economics, and environmental impacts [10]. The flue gas properties (such as composition, temperature, and pressure conditions) are also important parameters for selecting the appropriate process of CO2 separation [11]. The current scenario requires investments to mitigate carbon emissions effectively (CO2 capture and sequestration) [12]. Some primary CO2 capture techniques investigated globally are adsorption, absorption, chemical looping combustion, membrane separation, microbial/algal separation, hydrates-based separation, and cryogenic distillation method [2][3]. Figure 1 compares the advantages and disadvantages of different CO2 capture/separation techniques mentioned above. As shown in Figure 1, absorption, adsorption, and membrane-based separation methods offer high separation efficiency. Though cryogenic separation is the most mature technique, the process is highly energy intensive.
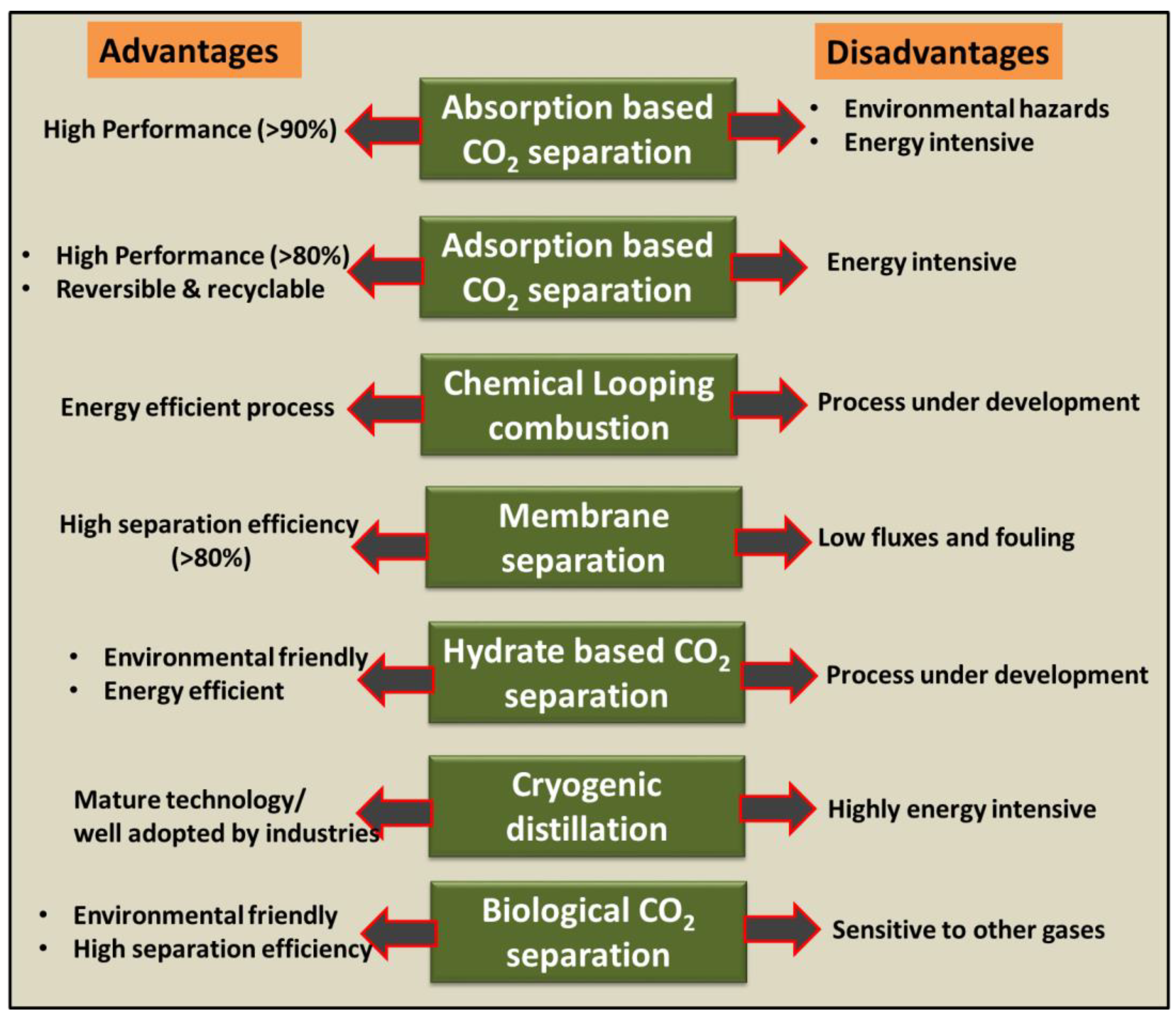
Figure 1. Comparison of different separation technologies for their advantages and disadvantages.
2.1. Absorption
The absorption method is widely applicable in the petroleum, coal, and natural-gas industries for separating CO2 [13]. Removing CO2 from a gas stream using different absorbents (physical and chemical) has been used in the industrial sector for over 50 years. The absorption method is broadly divided into two types: physical and chemical absorption [14][15]. Chemical absorption is the process by which a solvent absorbs CO2 and produces chemical compounds. These chemical components are later reused by removing the absorbed CO2 through different techniques. However, if the solvent is chemically inert, it does not interact with CO2. CO2 is chemically absorbed in two steps: the treated gas is initially introduced into counter-flowing interaction with the solvent stream. The solvent absorbs CO2 from the flue gas stream during this phase. As the solvent warms up, CO2 is desorbed in a stripping column, further migrating to the top of the column, where pure CO2 is recovered, compressed, and stored [14][16][17].
On the other hand, physical solvents do not undergo any reaction with CO2, making them more desirable for CO2 separation processes. Henry’s law of equilibrium in vapor–liquid mixtures governs the physical absorption process. It states that the relative gas pressure in equilibrium with the solvent at any given temperature is directly proportional to the amount of a gaseous phase dissolved in a unit volume of the solvent. Since the physical absorption process is pressure dependent, it performs better than chemical absorption at higher partial pressures of CO2, such as in an integrated gasification combined cycle (IGCC) power plants [14][18][19]. The coal, natural gas, and petroleum industries extensively use absorption techniques to segregate CO2 [13]. Kim and Yang [20] studied the competence of hollow-fiber membranes in CCS with various aqueous absorbents. The capability of the hollow polytetrafluoroethylene (PTFE) membrane filter was measured at varying temperatures. They conclude that the absorption rate of CO2 increases with the temperature rise. Sensitive absorbents such as 2-amino-2-methyl-l-propanol (AMP) and monoethanolamines (MEA) are vastly active agents to achieve augmented rates of CO2 absorption. Figure 2 shows the different categories of absorption medium broadly categorized into physical and chemical absorption. The physical and chemical processes are further divided into five subcategories: rectisol process, purisol process, selexol process, amine-based process, and inorganic chemical process.
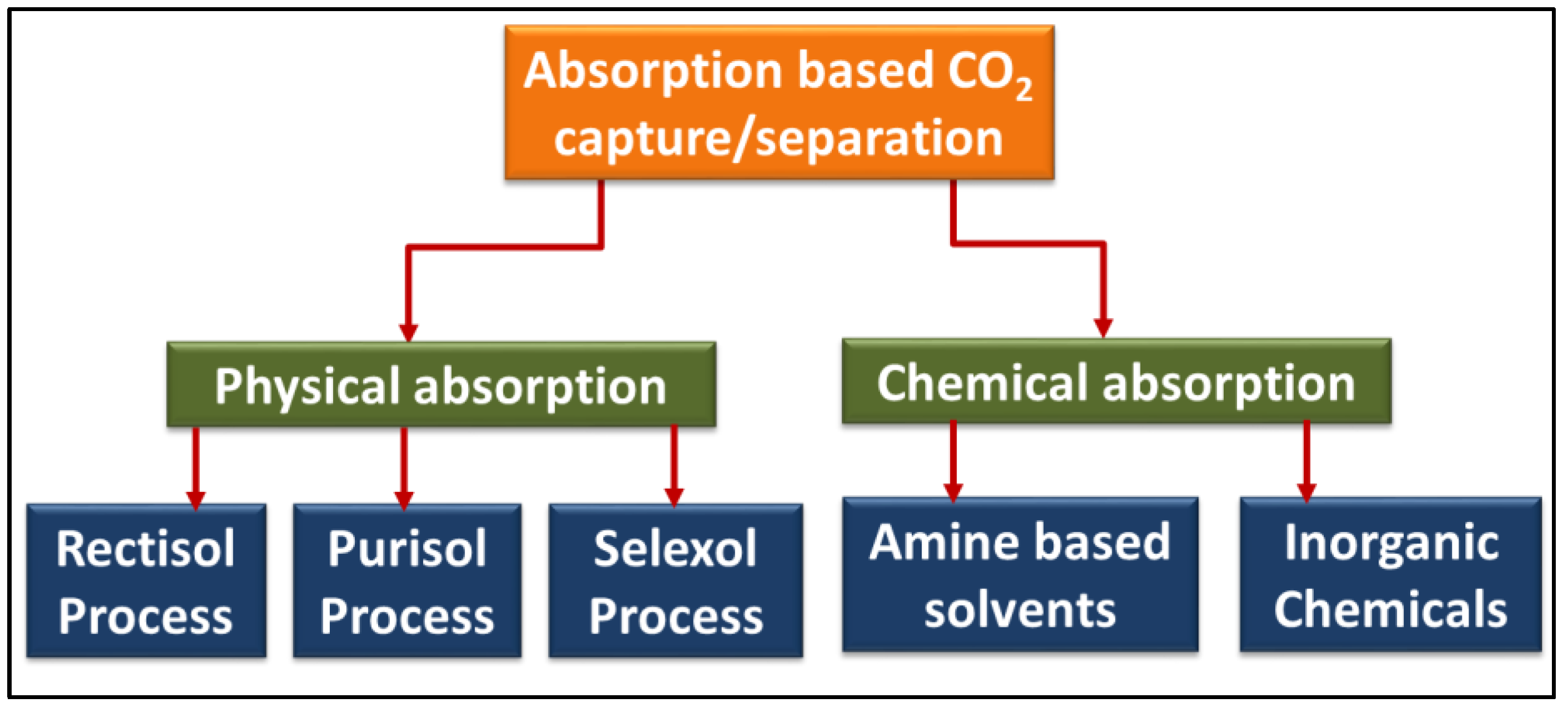
Figure 2. Absorption-based CO2 capture/separation processes.
In recent years, research has been undertaken on CO2 capture from fossil-fuel emission sources [21][22]. The CO2 absorption in aqueous media is evaluated by the equation [23]:
CO2 + 2H2O ↔ HCO3− + H3O+
Absorption of CO2 into aqueous solvent was initially done to purify gases such as synthetic gas, hydrogen, and natural gas [22][24]. However, research that originated on CO2 capture from fossil-fuel sources has been done with absorption [21][22]. CO2 absorption through the membrane is the combination of gas absorption and membrane separation processes. This method perceived a remarkable perspective in the last decade for capturing CO2 from flue gas streams [25].
The absorption process for the large implementation of CCS is the perhaps amine based which may cause equipment corrosion, solvent loss, and the production of volatile degradation constituents [26][27]. The release of nitramines and nitrosamines from the degradation of amine emissions can cause potential damage to human health [28]. Kozak et al. [29] presented a chilled-ammonia process (CAP) to capture CO2 from flue gases produced by different industries. They suggested that this process required low energy for the regeneration of CO2 at increased pressure and temperature, reducing the downstream compression, and is more environmentally friendly than the amine processes. Furthermore, the capacity of solvents in absorption was found to be better at lower temperatures, which necessitates the cooling of the solvent before the process and reducing the efficiency of the process [19]. Although absorption is the utmost developed CO2 separation process due to its high efficiency and low cost, it has certain environmental drawbacks due to the disposal of the absorbent after use [2].
2.2. Adsorption
Burning fossil fuels has led to the inexorable emission of greenhouse gases (GHG) and responsible for global warming. Carbon dioxide escape can be prevented by capturing it before it gets released into the environment. One such method that has been gaining popularity is the adsorption of CO2 on adsorbent material at high temperatures. CO2 adsorption at high temperatures is a significant CO2 separation method. Adsorption is a physical process in which a solid sorbent is used to fix the CO2 onto its surface. The adsorption process reduces energy consumption and cost during CO2 separation. Adsorbents can be used to capture single or multilayer gases depending on the absorbent’s temperature, pore size, surface force, and pressure [2][30][31]. The process employs an adsorbent with a nanoporous surface to precisely adsorb CO2 from the flue gas. Regeneration of the adsorbent is done by creating a vacuum environment around the adsorbent or by providing heat [32]. Generally used adsorbent materials are molecular sieves, zeolites, activated carbon, calcium oxides, lithium zirconate, and hydro-calcites [2]. Table 1 enlists different physical and chemical adsorbents used in the postcombustion capture of CO2.
| Sorbent | Operating Pressure (kPa) | Operating Temperature (K) | CO2 Capture Capacity (mol CO2/kg Sorbent) |
|---|---|---|---|
| CHEMICAL ADSORBENTS | |||
| Mesoporous (MgO) [34] | 101 | 298 | 1.8 |
| CaO nanopods [11] | 101 | 873 | 17.5 |
| CaO derived from nano-CaCO3 [11][34] | 101 | 923 | 16.7 |
| CaO-MgAl2O4 (spinel nanoparticles) [34] |
101 | 923 | 9.1 |
| Nano CaO/Al2O3 [34] | 101 | 923 | 6.0 |
| Lithium–Silicate [34] | - | 993 | 8.18 |
| CaO [34] | 100 | 873 | 17.3 |
| PHYSICAL ADSORBENTS | |||
| Activated Carbon [35] | 110 | 303 | 1.58 |
| NiO-ACs [35] | 101 | 298 | 2.227 |
| Na-Y [36] | 101.32 | 273 | 4.9 |
| NaKA [35] | 101.32 | 373 | 3.88 |
| MWNT [37] | 101 | 303 | 1.7 |
| CNT at (Cu3(btc)2) [36] | 1818 | 298 | 13.52 |
| MOF-177 [38] | 4545 | 298 | 33.5 |
| Pd-GNP Nanocomposite [39] | 1111 | 298 | 4.5 |
Adsorption is broadly categorized into chemical and physical adsorption processes. Chemical adsorption or ‘chemisorption’ is driven by chemical reactions at the contact surface. Metal salts and metal oxides are compounds that constitute most chemical adsorbents. ‘Physiosorption’ or physical adsorption does not affect the chemical structure of the adsorbent during adsorption. Inorganic porous materials such as zeolites, hydrotalcite, and activated carbons (AC) are widely used physical adsorbents [30][37][40][41]. Activated carbon is an economical material with a large surface area and flexible pore structure when treated with activating agents. However, effective CO2 separation through AC is possible when the AC possesses weak binding energy with carbon dioxide [42]. Zeolites are hydrophilic, yet strong CO2 adsorption agents. However, upon interaction with water, the strength of the links between the interconnected substances reduces, decreasing the adsorption capability of the zeolites. Applying metal–organic frameworks (MOFs) as adsorbents is a new approach. Metal ions or ion clusters are the essential components of MOFs, amalgamated by organic linkers and bridging agents to form stable coordination bonds. MOFs have advantages such as ease of synthesis and design with large porosity and modified pore features. Silica, a non-carbonaceous material, has a large surface area, small pore size, and high mechanical stability. Materials made of mesoporous silica use amine-based compounds to trap CO2 [40][42][43][44]. An effective adsorbent should have the following properties: (i) good mechanical strength, (ii) high sorption kinetics, (iii) high selectivity, and (iv) stable adsorption capacity [27].
The various pathways for carrying out the adsorption process are [13]:
- 1.
-
Pressure swing adsorption (PSA);
- 2.
-
Temperature swing adsorption (TSA);
- 3.
-
Electrical swing adsorption (ESA);
- 4.
-
Vacuum Swing Adsorption (VSA).
The recovery of CO2 captured during the adsorption process through processes such as pressure-temperature swing adsorption (PTSA), vacuum swing adsorption (VSA), pressure swing adsorption (PSA), and temperature swing adsorption (TSA) processes where PSA and TSA are the most widely used techniques. PSA adsorbs CO2 onto a solid adsorbent surface at fluctuating pressures between maximum and minimum permissible pressure limits. TSA is the process of CO2 recovery through variations of temperature using hot air or steam. The PSA method is favorably implemented in industrial applications due to its high recovery efficiency (85%) and lower application cost than TSA. However, TSA is observed to be 95% effective in recovering CO2 from adsorbed surfaces, although it has a longer regeneration time than PSA. The requirement of high temperature during TSA and high energy during PSA methods are the most significant drawbacks of these two methods [6][7][45][46]. Yong et al. [47] reviewed the various adsorbents at high temperatures. They studied material from carbon-based adsorbents with high adsorption capacity for CO2 separation at surrounding temperature and pressure to other agents such as zeolites, metal oxide sorbents, and hydrotalcite-like compounds (HTlcs). It is of the utmost importance to understand that the choice of adsorbents depends on the operating conditions of the process. MgAl2O4, CaO– and nano CaO/Al2O3 are the most effective chemical adsorbents. The regeneration of chemical adsorbents is complex, even though they have high adsorption capacity and selectivity [30].
References
- International Energy Agency. Global Energy Review: CO2 Emissions in 2021 Global Emissions Rebound Sharply to Highest Ever Level; International Energy Agency: Paris, France, 2021.
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443.
- Bhown, A.S.; Freeman, B.C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632.
- Pandey, G.; Poothia, T.; Kumar, A. Hydrate based carbon capture and sequestration (HBCCS): An innovative approach towards decarbonization. Appl. Energy 2022, 326, 119900.
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348.
- Takamura, Y.; Aoki, J.; Uchida, S.; Narita, S. Application of high-pressure swing adsorption process for improvement of CO2 recovery system from flue gas. Can. J. Chem. Eng. 2001, 79, 812–816.
- Clausse, M.; Merel, J.; Meunier, F. Numerical parametric study on CO2 capture by indirect thermal swing adsorption. Int. J. Greenh. Gas Control 2011, 5, 1206–1213.
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282.
- Erlach, B.; Schmidt, M.; Tsatsaronis, G. Comparison of carbon capture IGCC with pre-combustion decarbonisation and with chemical-looping combustion. Energy 2011, 36, 3804–3815.
- Wong, S.; Bioletti, R. Carbon Dioxide Separation Technologies. Available online: http://www.ipcc.ch/ (accessed on 23 November 2022).
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131.
- Simpson, A.P.; Simon, A.J. Second law comparison of oxy-fuel combustion and post-combustion carbon dioxide separation. Energy Convers. Manag. 2007, 48, 3034–3045.
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441.
- Rackley, S.A. Absorption capture systems. In Carbon Capture and Storage, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–185.
- Sifat, N.S.; Haseli, Y. A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation. Energies 2019, 12, 4143.
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663.
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769.
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187.
- Figueroa, J.D.; Fout, T.; Plasynski, S.I.; McIlvried, H.; Srivastava, R.D. Advances in CO2 Capture Technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20.
- Kim, Y.-S.; Yang, S.-M. Absorption of carbon dioxide through hollow fiber membranes using various aqueous absorbents. Sep. Purif. Technol. 2000, 21, 101–109.
- Lee, S.C.; Choi, B.Y.; Lee, T.J.; Ryu, C.K.; Ahn, Y.S.; Kim, J.C. CO2 absorption and regeneration of alkali metal-based solid sorbents. Catal. Today 2006, 111, 385–390.
- Hu, G.; Nicholas, N.J.; Smith, K.H.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into promoted potassium carbonate solutions: A review. Int. J. Greenh. Gas Control 2016, 53, 28–40.
- Bosch, H.; Versteeg, G.F.; Van Swaaij, W.P.M. Kinetics of the reaction of CO2 with the sterically hindered amine 2-Amino-2-methylpropanol at 298 K. Chem. Eng. Sci. 1990, 45, 1167–1173.
- Shrier, A.L.; Danckwerts, P.V. Carbon Dioxide Absorption into Amine-Promoted Potash Solutions. Ind. Eng. Chem. Fundam. 1969, 8, 415–423.
- Feron, P.H.; Jansen, A. The production of carbon dioxide from flue gas by membrane gas absorption. Energy Convers. Manag. 1997, 38, S93–S98.
- Bougie, F.; Iliuta, M.C. CO2 Absorption in Aqueous Piperazine Solutions: Experimental Study and Modeling. J. Chem. Eng. Data 2011, 56, 1547–1554.
- Fredriksen, S.B.; Jens, K.-J. Oxidative Degradation of Aqueous Amine Solutions of MEA, AMP, MDEA, Pz: A Review. Energy Procedia 2013, 37, 1770–1777.
- da Silva, C.F.N.; Dias, A.P.B.; Santana, A.P.R.; Pizzo, J.S.; de Souza, F.M.; Lazarin, A.M.; Sernaglia, R.L.; Andreotti, E.I.S. Retraction: Intercalation of Amines into Layered Calcium Phosphate and Their New Behavior for Copper Retention from Ethanolic Solution. Open J. Synth. Theory Appl. 2013, 2, 1–7.
- Kozak, F.; Petig, A.; Morris, E.; Rhudy, R.; Thimsen, D. Chilled ammonia process for CO2 capture. Energy Procedia 2009, 1, 1419–1426.
- Songolzadeh, M.; Ravanchi, M.T.; Soleimani, M. Carbon Dioxide Capture and Storage: A General Review on Adsorbents. Int. J. Chem. Mol. Eng. 2012, 6, 906–913.
- Meisen, A.; Shuai, X. Research and development issues in CO2 capture. Energy Convers. Manag. 1997, 38, S37–S42.
- Liu, H.; Liu, B.; Lin, L.-C.; Chen, G.; Wu, Y.; Wang, J.; Gao, X.; Lv, Y.; Pan, Y.; Zhang, X.; et al. A hybrid absorption–adsorption method to efficiently capture carbon. Nat. Commun. 2014, 5, 5147.
- Mishra, A.K.; Ramaprabhu, S. Palladium nanoparticles decorated graphite nanoplatelets for room temperature carbon dioxide adsorption. Chem. Eng. J. 2012, 187, 10–15.
- Abid, H.R.; Pham, G.H.; Ang, H.-M.; Tade, M.O.; Wang, S. Adsorption of CH4 and CO2 on Zr-metal organic frameworks. J. Colloid Interface Sci. 2012, 366, 120–124.
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854.
- Jang, D.-I.; Park, S.-J. Influence of nickel oxide on carbon dioxide adsorption behaviors of activated carbons. Fuel 2012, 102, 439–444.
- Anbia, M.; Hoseini, V. Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem. Eng. J. 2012, 191, 326–330.
- Lee, Z.H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Post-combustion carbon dioxide capture: Evolution towards utilization of nanomaterials. Renew. Sustain. Energy Rev. 2012, 16, 2599–2609.
- Chiao, C.-H.; Chen, J.-L.; Lan, C.-R.; Chen, S.; Hsu, H.-W. Development of carbon dioxide capture and storage technology—Taiwan power company perspective. Sustain. Environ. Res. 2011, 21, 1–8.
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823.
- Dantas, T.L.P.; Luna, F.M.T.; Silva, I.J., Jr.; Torres, A.E.B.; Azevedo, D.C.S.; Rodrigues, A.E.; Moreira, R.F.P.M. Carbon dioxide–nitrogen separation through pressure swing adsorption. Chem. Eng. J. 2011, 172, 698–704.
- Bolland, P.O.; Nord, P.L.O. Carbon Dioxide Emission Management in Power Generation; Wiley: New York, NY, USA, 2020.
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189.
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849.
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645.
- Ritter, J.A. Radically New Adsorption Cycles for Carbon Dioxide Sequestration. In Proceedings of the University Coal Research Contractors Review Meeting, Pittsburgh, PA, USA, 2–3 June 2004.
- Yong, Z.; Mata, V.; Rodrigues, A. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
15 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




