
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | J. Carlos Menéndez | -- | 2104 | 2023-08-07 07:31:14 | | | |
| 2 | Jason Zhu | Meta information modification | 2104 | 2023-08-08 03:42:13 | | |
Video Upload Options
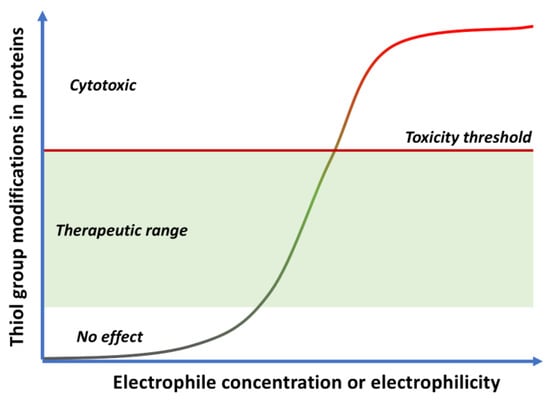
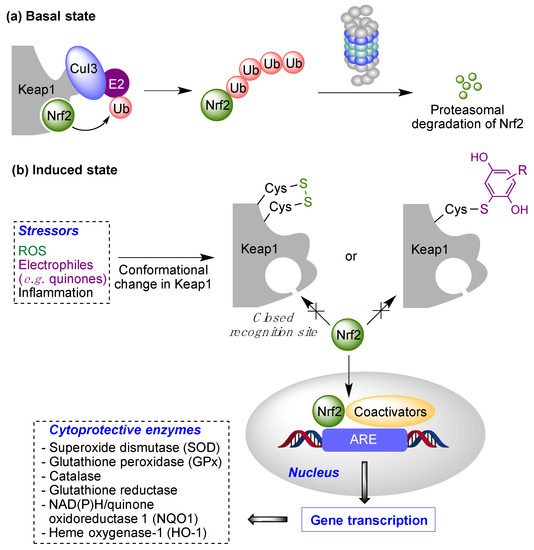
Quinones can in principle be viewed as a double-edged sword in the treatment of neurodegenerative diseases, since they are often cytoprotective but can also be cytotoxic due to covalent and redox modification of biomolecules. Nevertheless, low doses of moderately electrophilic quinones are generally cytoprotective, mainly due to their ability to activate the Keap1/Nrf2 pathway and thus induce the expression of detoxifying enzymes. Some natural quinones have relevant roles in important physiological processes. One of them is coenzyme Q10, which takes part in the oxidative phosphorylation processes involved in cell energy production, as a proton and electron carrier in the mitochondrial respiratory chain, and shows neuroprotective effects relevant to Alzheimer’s and Parkinson’s diseases. Additional neuroprotective quinones that can be regarded as coenzyme Q10 analogues are idobenone, mitoquinone and plastoquinone.
1. General Features of Neurodegenerative Diseases
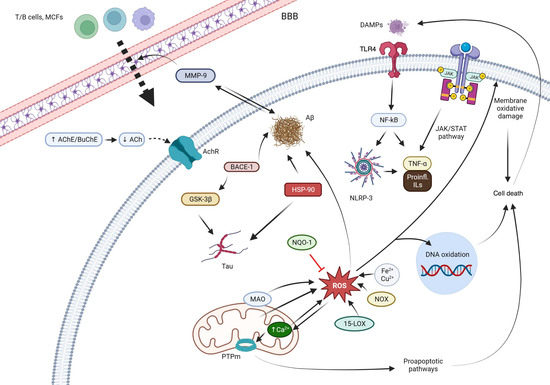
2. General Features of Quinone Chemistry Relevant to Neuroprotection
- (a)
-
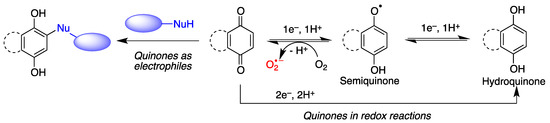
Quinones are redox active molecules that can form semiquinone radicals by a reversible one-electron transfer process that in the presence of oxygen generates superoxide, a reactive oxygen species in the reverse reaction (Figure 2). Thus, quinones can cause oxidative stress leading to the oxidation of lipids, proteins and DNA.
- (b)
-
Quinones are Michael acceptors and thus can cause cellular damage by alkylating cellular proteins, especially at cysteine residues, or DNA (Figure 2).
- (c)
-
Due to their planar structure and their ability to form hydrogen bonds, many quinones are able to bind to amyloid proteins, preventing their aggregation and allowing the visualization of diffuse and dense-core amyloid plaques [12]. Molecular dynamics simulations have shown that anthraquinone, used as a model simple quinone, interferes with the early phase of aggregation by intercalation into the β-sheet of the hydrophobic H14QKLVFF20 sequence that promotes Aβ self-assembly. The amyloid-quinone complex is formed via polar interactions with the peptide backbone, which destabilize interstrand hydrogen bonds [13].
3. Quinones as Redox Modifiers of Biomolecules
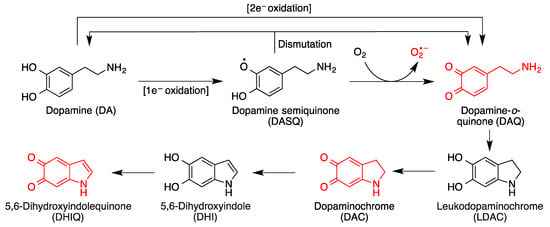
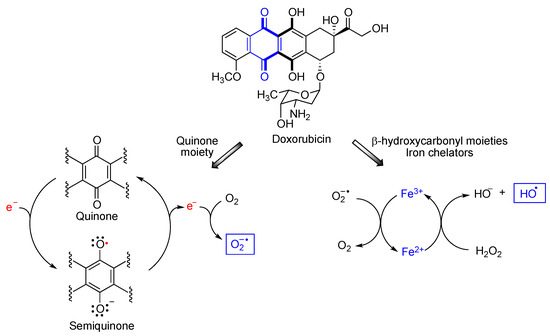
4. Quinones as Covalent Modifiers of Biomolecules
References
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118.
- Dokholyan, N.V.; Mohs, R.C.; Bateman, R.J. Challenges and progress in research, diagnostics, and therapeutics in Alzheimer’s disease and related dementias. Alzheimer’s Dement. Transl. Res. Clin. 2022, 8, e12330.
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714.
- Armstrong, R. What causes neurodegenerative disease? Folia Neuropathol. 2020, 58, 93–112.
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325.
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376.
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, 18.
- Cummings, J. Disease modification and neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017, 6, 25.
- Dahlem, M.A.; Nguema Edzang, R.W.; Catto, A.L.; Raimundo, J.-M. Quinones as an efficient molecular scaffold in the antibacterial/antifungal or antitumoral arsenal. Int. J. Mol. Sci. 2022, 23, 14108.
- Subhasmita Sahoo, P.M.; Behera, S.; Behura, R.; Acharya, A.; Biswal, D.; Kumar Suna, S.; Shaoo, R.; Soren, R.C.; Jali, B.R. A brief review: Antibacterial activity of quinone derivates. Biointerface Res. Appl. Chem. 2022, 12, 3247–3258.
- Ferreira, V.F.; de Carvalho, A.S.; Ferreira, P.G.; Lima, C.G.; Silva, F.D.C.D. Quinone-based drugs: An important class of molecules in medicinal chemistry. Med. Chem. 2021, 17, 1073–1085.
- Shin, N.N.; Jeon, H.; Jung, Y.; Baek, S.; Lee, S.; Yoo, H.C.; Bae, G.H.; Park, K.; Yang, S.-H.; Han, J.M.; et al. Fluorescent 1,4-naphthoquinones to visualize diffuse and dense-core amyloid plaques in APP/PS1 transgenic mouse brains. ACS Chem. Neurosci. 2019, 10, 3031–3044.
- Convertino, M.; Pellarin, R.; Catto, M.; Carotti, A.; Caflisch, A. 9,10-Anthraquinone hinders β-aggregation: How does a small molecule interfere with Aβ-peptide amyloid fibrillation? Prot. Sci. 2009, 18, 792–800.
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527.
- Pavlin, M.; Repič, M.; Vianello, R.; Mavri, J. The Chemistry of neurodegeneration: Kinetic data and their implications. Mol. Neurobiol. 2016, 53, 3400–3415.
- Biosa, A.; Arduini, I.; Soriano, M.E.; Giorgio, V.; Bernardi, P.; Bisaglia, M.; Bubacco, L. Dopamine oxidation products as mitochondrial endotoxins, a potential molecular mechanism for preferential neurodegeneration in Parkinson’s disease. ACS Chem. Neurosci. 2018, 9, 2849–2858.
- Zhang, S.; Wang, R.; Wang, G. Impact of dopamine oxidation on dopaminergic neurodegeneration. ACS Chem. Neurosci. 2019, 10, 945–953.
- Liu, J.; Song, E.; Liu, L.; Ma, X.; Tian, X.; Dong, H.; Song, Y. Polychlorinated biphenyl quinone metabolites lead to oxidative stress in HepG2 cells and the protective role of dihydrolipoic acid. Toxicol. In Vitro 2012, 26, 841–848.
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014, 209, 56–67.
- Avendaño, C.; Menéndez, J.C. Anticancer strategies involving radical species. In Medicinal Chemistry of Anticancer Drugs, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 4.
- Cole, M.P.; Chaiswing, L.; Oberley, T.D.; Edelmann, S.E.; Piascik, M.T.; Lin, S.M.; Kiningham, K.K.; St Clair, D.K. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc. Res. 2006, 69, 186–197.
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708.
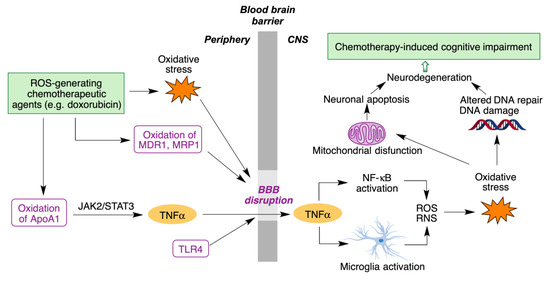
- Rena, X.; Boriero, D.; Chaiswing, L.; Bondada, S.; St. Clair, D.K.; Butterfield, D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive T impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1088–1097.
- Singh, J. The ascension of targeted covalent inhibitors. J. Med. Chem. 2022, 65, 5886–5901.
- Bolton, J.L.; Dunlap, T.L. Formation and biological targets of quinones: Cytotoxic versus cytoprotective effects. Chem. Res. Toxicol. 2017, 30, 13–37.
- Bolton, J.L.; Dunlap, T.L.; Dietz, B.M. Formation and biological targets of botanical o-quinones. Food Chem. Toxicol. 2018, 120, 700–707.
- Birringer, M. Hormetics: Dietary triggers of an adaptive stress response. Pharm. Res. 2012, 28, 2680–2694.
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20.
- Cores, A.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 regulation processes as a source of potential drug targets against neurodegenerative diseases. Biomolecules 2020, 10, 904.
- Satoh, T.; Rezaie, T.; Seki, M.; Sunico, C.R.; Tabuchi, T.; Kitagawa, T.; Yanagitai, M.; Senzaki, M.; Kosegawa, C.; Taira, H.; et al. Dual neuroprotective pathways of a pro-electrophilic compound via HSF-1-activated heat-shock proteins and Nrf2-activated phase 2 antioxidant response enzymes. J. Neurochem. 2011, 119, 569–578.
- Baell, J.B. Feeling Nature’s PAINS: Natural products, natural product drugs, and pan assay interference compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628.
- Capuzzi, S.J.; Muratov, E.N.; Tropsha, A. Phantom PAINS: Problems with the utility of alerts for Pan-Assay INterference Compounds. J. Chem. Inf. Model. 2017, 57, 417–427.




