Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Azizur Rahman | -- | 2897 | 2023-07-21 00:10:35 | | | |
| 2 | Jessie Wu | -41 word(s) | 2856 | 2023-07-21 07:13:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rahman, A. Bacteriophages in Agriculture. Encyclopedia. Available online: https://encyclopedia.pub/entry/47088 (accessed on 07 February 2026).
Rahman A. Bacteriophages in Agriculture. Encyclopedia. Available at: https://encyclopedia.pub/entry/47088. Accessed February 07, 2026.
Rahman, Azizur. "Bacteriophages in Agriculture" Encyclopedia, https://encyclopedia.pub/entry/47088 (accessed February 07, 2026).
Rahman, A. (2023, July 20). Bacteriophages in Agriculture. In Encyclopedia. https://encyclopedia.pub/entry/47088
Rahman, Azizur. "Bacteriophages in Agriculture." Encyclopedia. Web. 20 July, 2023.
Copy Citation
Bacteriophages, more commonly referred to as phages, are a class of viruses discovered for their bactericidal effects even before the discovery of penicillin and other antibiotics. An increasingly popular alternative to antibiotics is bacteriophages to control bacterial diseases. Their unique bactericidal properties make them an ideal alternative to antibiotics, as many countries begin to restrict the usage of antibiotics in agriculture. The recent evidence from within the past decade on the efficacy of phage therapy on common foodborne pathogens are analyzed, namely, Escherica coli, Staphylococcus aureus, Salmonella spp., and Campylobacter jejuni.
antimicrobial resistance (AMR)
Bacteriophages
Agriculture
food-borne pathogens
1. Bactericidal Effects of Bacteriophages Demonstrated in Agriculture
Bacteriophages kill bacteria through their lytic replication cycle, where the release of phage progeny results in lysis of the bacterial membrane and subsequent death of the bacterium. Phages have been proven extensively in vitro to exhibit significant bacterial inhibition and bactericidal effects against various disease-causing bacteria within humans and agricultural animals [1][2][3][4][5][6][7]. In vitro studies have demonstrated that combining several phages into cocktails increased bactericidal effectiveness and the range against multiple serotypes of a bacterial species, resulting in increased effectiveness in clearing bacterial populations. A practical application of a bacteriophage cocktail was demonstrated in vitro in which they assessed the therapeutic efficacy of a cocktail of three E. coli phages, vB_EcoM_SYGD1, vB_EcoP_SYGE1 and vB_EcoM_SYGMH1, in treating cow mastitis caused by drug-resistant E. coli [8]. The phages used in the cocktail were stable at various temperatures, pH values, and chloroform values, demonstrating possible candidates for phage therapy [8]. The benefits of phage cocktails are a reduction in the development of phage resistance, improvement in symptoms, antimicrobial activity and similar effects to antibiotics when applied.
Multiple in vivo studies of phage therapy using animal models have also been conducted in recent years. These findings highlight not only the specificity of phages to specific bacteria, avoiding unintended target cells but also the stability of phages within different environments and conditions. It is ideal for oral administration in capsules [5][9]. This adaptability and survivability of phages in different conditions may also reflect their natural environments from which most phages are found and isolated; for example, several phage strains were found and isolated from swine-fecal sewage as well as wastewater from poultry slaughterhouses. Their effectiveness against multiple strains of S. aureus (18 strains) and E. coli (3 strains) was demonstrated in vivo in mice and broiler chickens, clearing infections of MRSA S. aureus and E. coli colibacillosis [5][10]. Another phage strain isolated from the natural environment of the Ganges River was found to have a broad host range of 31 different E. coli strains and serotypes. It was able to demonstrate effective bactericidal effects in reducing E. coli populations within an in vivo mouse model. This phage strain exhibited 100% bacterial clearance when three doses were given at 6-h intervals [11] (Figure 1). Effective and specific-targeting phages can be readily isolated from nearby natural environments and cultivated to create doses of phage treatments, and with advances in molecular and gene editing techniques, phage characteristics and virulence can be altered and enhanced more easily than the development of new classes of antibiotics.
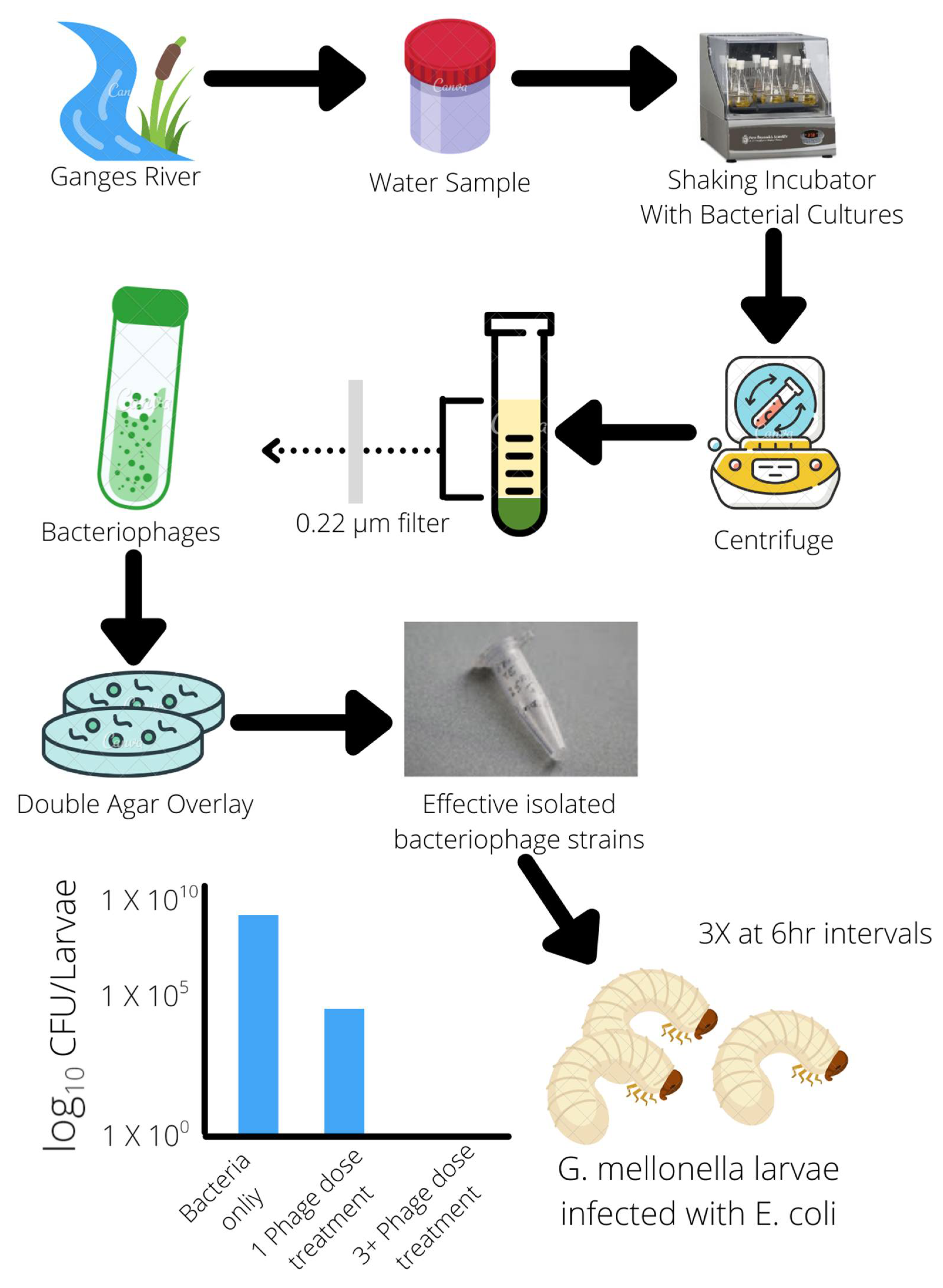
Figure 1. Isolation of bacteriophages from the natural environment effective against E. coli.
As mentioned earlier, several countries have approved the use of several phage products in agriculture and food processing. Recent studies have shown high success in the ability of phages to suppress and control pathogenic bacterial populations within farms and processing facilities. Several phage cocktails have been made against several common species of food-borne bacteria, for example, EcoShieldTM against E. coli, Listex P100TM against Listeria, and SalmoFREETM against Salmonella. These phage cocktails have been studied at various points of the food supply chain, from Salmonella in commercial poultry farms to processing plants for ready-to-eat foods. All have demonstrated an effective ability to control bacterial populations in livestock and reduce pathogens in food products to legal standards [12][13][14]. The UK and USA have deemed phages “safe for consumption”, and the USA has even allowed certain phage products against Listeria, Salmonella, and E. coli to be used as food preservatives and be applied directly to food products [15]. However, problems can arise with unregulated use and lack of veterinary surveillance, similar to antibiotics. While predicted to be capable of reducing and clearing bacterial concentrations in contaminated foods, as mentioned in a report by the EFSA Panel on Biological Hazards, with unmonitored usage of phage products and in unspecified doses, the ability of the phage products to reduce bacterial populations in food products can become more varied and unreliable. Without the proper determination of bacterial concentrations by professionals and veterinarians, the applied dosage of the phage products may sometimes be insufficient in clearing bacterial populations and may not be sufficient to prevent recontamination; thus, it can only be currently used as a processing aid [13]. A major benefit is that the possibility of phage resistance and persistence in the environments of processing facilities is considerably low and even minimal when paired with proper disinfection protocols and adequate disposal of unsold treated food products [15][16]. While current phage products may possess certain shortcomings, their ability to reduce pathogenic bacteria in the food supply chain is undisputed. As more data and further research are done to address these issues, phage cocktails can be a promising aid or replacement for antibiotic use in agriculture and the food supply chain.
Plants are known to suffer infections from pests, fungi, and bacteria. Thus, in addition to pesticides and antimicrobials, common antibiotics are used to combat bacterial infections. As with livestock bacterial pathogens, a variety of common antibiotics used in humans are also used to combat plant pathogens, namely, Pseudomonas spp. and Erwinia spp. Strains from both species were found to possess resistance to high levels of streptomycin (1000 micrograms/mL) [17] and tomato-infecting strains of Pseudomonas spp. possess additional resistance to ampicillin and chloramphenicol [18]. With a high rate of AMR in Pseudomonas spp. observed in recent years, the risk for blight outbreaks in fruit crops is ever increasing; thus, the usage of phage therapy can serve to mitigate this issue and bypass any current resistance mechanisms employed by Pseudomonas spp. In vitro experiments with infected kiwi and tomato plants have shown rapid decreases in bacterial populations and symptoms, including damage to leaf tissues [19][20]. Furthermore, phages have shown additional benefits over antibiotics, as they exhibit a wide tolerance to environmental conditions [19] and selective targeting, avoiding lysis of beneficial Pseudomonas strains [20].
Soft rot Enterobacteriaceae (SRE) infections cause blackleg and soft rot diseases, significantly decreasing crop production and yield. T4-related LIMEstone phages infecting D. solani were isolated and showed reduced disease incidence and severity, as well as higher yields in laboratory assays and in field experiments [21]. Their experiment showed that LIMEstone phages were very effective at rapidly infecting all D. solani strains [21]. A close relative of LIMEstone1, ΦD5, was tested and remained viable in severe environmental conditions previously unsuitable for phage therapy [22]. Phage treatment of tissue culture and compost with ΦD5 resulted in high levels of protection against infection in potato crops. ΦD5 was shown to have the potential to be used as a biological control measure against Dickeya spp. caused soft rot and blackleg [22].
A cocktail of 46 new bacteriophages was created to be used for biocontrol of D. solani, and their efficacy in treating soft rot in potatoes under simulated storage conditions was observed [23]. They showed that phage treatment significantly lowered soft rot disease incidence and severity. supporting the use of a phage cocktail in reducing and controlling D. solani populations and its spread in potato crops [23]. With further in vivo studies and field trials, phage therapy demonstrates potential in treating bacterial crop diseases. Biochar increases phage adsorption of antibiotic-resistant E. coli and Pseudomonas aeruginosa bacteria by adsorbing the bacteria in the biochar, increasing the bacterial density and the bactericidal potential of the polyvalent phages [24]. Combined biochar and polyvalent phage treatment reduced residual levels of K-12 and PAO1 and significantly reduced accumulative levels of ARGs in the roots and leaves of lettuce, improving lettuce quality. An added benefit of the combined treatment is that the addition of biochar was associated with an increase in microbial biodiversity in soil and lettuce and diversity of beneficial soil bacteria [24].
Bacteria employ a variety of defense mechanisms against antibiotics, including producing biofilms and evasion by presiding within host cells. Bacteria produce an extracellular matrix that binds multiple bacteria together into a cooperative community and provides structural stability and a layer of defense that confers resistance to recognition from the host’s immune cells and a multitude of antibiotics. This all resulted in the need for a combination of antibiotics and increased dosages. While biofilms may provide some resistance against phage recognition, some phages isolated from natural water sources have coevolved the ability to penetrate biofilms and infect the underlying bacteria. With optimization in vitro, biofilm production can be inhibited or prevented and even completely degraded within a bacterial population [7]. Studies have begun to suggest the use of phages that produce specialized enzymes that degrade biofilms. These phages can be supplemented with minimal doses of antibiotics to remove the remaining extracellular and intracellular populations of multidrug-resistant bacteria [25]. Phages were found to have the ability to clear bacteria that reside within infected cells, as demonstrated by phage vB_SauM_JS25 clearance of intracellular populations of S. aureus from within bovine mammary epithelial cells in vivo [26], providing treatments for persistent agricultural epidemics, which would otherwise require large amounts of strong antibiotics to resolve. Furthermore, biofilms may increase susceptibility against phages, as the clustering of similar clonal bacteria and the dynamics of fluids to carry phages to biofilms may result in increased phage interaction due to the increased size, creating a more accessible target for the phages than individual bacteria [27]. The ability of biofilms to allow bacterial populations to adhere to surfaces and resist a certain degree of chemical and mechanical stress is increasingly problematic in food-borne pathogens that can adhere to surfaces within food processing facilities. Phages can effectively reduce biofilm and bacterial populations that adhere to stainless steel surfaces [28], making them ideal as an aid for reducing bacterial contamination in addition to proper disinfection protocols.
Endolysins are bacteriophage-encoded enzymes that hydrolyze the host cell wall through peptidoglycan degradation and allow for the release of bacteriophage progenies. They are vital for the lytic phage life cycle to occur and in recent years show promise as an alternative to antibiotics. This enzyme has been a topic of focus in sectors, such as food, biotechnology, and human medicine with practical applications in biofilm eradication and antimicrobial function [29]. Resistance to endolysins by bacteria may occur through peptidoglycan modifications or bacterial inhibitor proteins; however, the possibility of this development is rare and has not yet been shown in vitro [30]. Endolysins from phages λSA2 and B30 were found to work synergistically against Streptococci in vitro and in milk [31]. In whole milk, λSA2 endolysins showed stronger lytic activity than B30 endolysins against all three Streptococcus species used in the experiment [31]. However, λSA2 and B30 endolysins do not have synergistic abilities in mastitis treatment, whereas, with individual endolysin treatment, bacterial concentrations of all three Streptococcal species were significantly reduced in the mammary gland [31]. Additional research was performed to engineer a unique enzyme through the removal of the middle amidase domain in LysK, termed LysKΔamidase, and showed strong evidence of antimicrobial activity and biofilm eradication that phage lysin can have applied in vitro [32]. They showed that LysKΔamidase had high activity against S. aureus and lytic activity against live MRSA strains as well as methicillin-susceptible S. aureus. LysKΔamidase is also safer to apply to the animal body than LysK, and it is very effective in eradicating biofilms produced by MRSA [32]. Thus, phage therapy has been demonstrated to be effective against agricultural pathogenic bacteria and, in some cases, exhibits additional qualities to treat infections that not even traditional antibiotic therapy can.
2. Advantages of Phage Therapy over Conventional Antibiotics
One main challenge of phage therapy is how readily available the technology is to be used in practical settings. Phages are a broad classification and often have different optimal functioning conditions and storage condition requirements, such as pH, temperature, and storage media. Several recently discovered and isolated phages, such as pSa-3 and a three E. coli phage cocktail comprised of vB_EcoM_SYGD1, vB_EcoP_SYGE1 and vB_EcoM_SYGMH, can survive at high temperatures and pH and stability at various temperatures, pH values and chloroform values, respectively [8]. The broad-spectrum activity of endolysins, such as LysKΔamidase and its broad pH range of 3 to 11 also make it ideal for a variety of applications [32]. Phage banks have recently been established as long-term storage of phages, allowing timely revival for research and application. However, varying tolerances to freezing temperatures and growth media and long-term storage of phages pose a potential problem to both purities of the isolates and may decrease the viability by up to 20% under improper glycerol storage conditions [33].
However, phages do exhibit an advantageous characteristic over antibiotics, which is the ability to work synergistically with other compounds. This was further demonstrated when phage pSa-3 combined with a surfactant, Tween 20, was tested against S. aureus aggregates in vitro and in vivo. Tween 20 was found to have prevented S. aureus aggregation and increased the adsorption rate and biofilm degradation ability of phage pSa-3 [34]. This suggests that there are accessory compounds that can be administered alongside phage therapy to increase effectiveness, as opposed to antibiotic therapies that increase effectiveness by including more classes of antibiotics.
Recent discoveries found phage’s potential for biopreservation to extend shelf life for food at risk of spoilage due to pathogenic bacteria [35]. The conventional method of biopreservation can be faulty, as they are unreliable in protecting foods from decay caused by bacteria and run the risk of possible pathogens transferring to the consumer, but phage products can be designed to be stable at various temperatures and control bacterial population growth. A study looking at the effects of biopreservation in chilled fish discovered that traditional methods caused higher incidences of alimentary infections and led to the rapid formation of AMR in the fish. When bacteriophage cocktails were used in biopreservation, bacterial degradation was delayed by up to 3 days longer than conventional methods [36]. It was also observed that chilled pork exposed to phage treatment had significantly reduced Salmonella populations while also reducing odor and extending the shelf life of the pork up to 14 days [37]. Through rigorous testing, it can be determined that the shelf life can be extended using FDA-approved phages versus temperate phages that could change the bacterial genome without killing them, which would run the risk of further AMR [38][39].
Antibiotics used at subtherapeutic levels improve growth rate and efficiency and improve reproductive performance while also reducing mortality and morbidity [40][41]. Higher intermediate levels have been key to preventing diseases, and even higher therapeutic levels can treat diseases in animals, which is why they are so integral for many feeding programs [40][41]. However, a common theme when talking about antibiotics is antibiotic resistance. Due to the importance of antibiotics to various industries, the decreased effectiveness of antibiotic treatment would be disastrous, as the world already shifts to a “post-antibiotic era” [42][43].
As mentioned previously, bacteriophages are highly selective and will only reduce target bacterial populations, thus ignoring beneficial commensal bacteria residing in the microbiome of livestock. In addition to different bacterial families, the host range of phages can be specified to differentiate between pathogenic and nonpathogenic strains within the same bacterial species [44]. Phage therapy can directly affect pathogens without any side effects to the microbiome, but antibiotics can conversely cause collateral damage as they disrupt the microbiome and surrounding structures [42][43]. This selectivity was further demonstrated in mouse models, in which only foodborne bacteria, such as E. coli, Salmonella, and Listeria were targeted, while the rest of the microbiome was unaffected [45]. In recent decades, many individuals have been found to develop an allergic response to antibiotics, to which phages can act as an alternative to many who are unable to receive antibiotic therapy [42][43]. This has further safety benefits, as phages are unable to target mammalian cells and thus will not have a direct effect [43]. Phage therapy demonstrates much more targeted treatment than antibiotics, which exhibit indiscriminate elimination of bacteria, including commensal bacteria necessary for the health of many livestock animals.
The increase in new bacteriophage research demonstrates the efficacy of phage cocktails in reducing bacterial populations in the environment and livestock animals. Phage therapy is a good potential alternative to antibiotics sub-therapeutically, since the phage targets a host bacterium and is harmless with virtually no adverse effects or changes to the gut commensal bacteria in animals [10][46][47]. Sub therapeutically, phage therapy is capable of disease prevention, while reversing body weight loss associated with E. coli infection [10] and phage cocktails were found to have a synergistic effect with probiotics in improving the average daily feed intake and weight gain of pigs, suggesting a potential for phage therapy to replace antibiotics as growth promoters [48][49][50]. Hence, phages can provide benefits over traditional antibiotics, such as the ability to bypass biofilms and overcome acquired phage resistance, making them great candidates for the agricultural sector and even the medical sector to overcome this crisis of high antibiotic resistance.
References
- Capparelli, R.; Nocerino, N.; Iannaccone, M.; Ercolini, D.; Parlato, M.; Chiara, M.; Iannelli, D. Bacteriophage therapy of Salmonella enterica: A fresh appraisal of bacteriophage therapy. J. Infect. Dis. 2010, 201, 52–61.
- Cerveny, K.E.; DePaola, A.; Duckworth, D.H.; Gulig, P.A. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 2002, 70, 6251–6262.
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773.
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F.; et al. Development of a bacteriophage cocktail to constrain the emergence of phage-resistant Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 327.
- Wang, Z.; Zheng, P.; Ji, W.; Fu, Q.; Wang, H.; Yan, Y.; Sun, J. SLPW: A virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2016, 7, 934.
- Porter, J.; Anderson, J.; Carter, L.; Donjacour, E.; Paros, M. In vitro evaluation of a novel bacteriophage cocktail as a preventative for bovine coliform mastitis. J. Dairy Sci. 2016, 99, 2053–2062.
- Yulinery, T.; Triana, E.; Suharna, N.; Nurhidayat, N. Isolation and anti-Escherichia coli biofilm activity of lytic bacteriophages isolated from water environment in vitro. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2019; Volume 308, p. 012010.
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell. Infect. Microbiol. 2021, 11, 555.
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023.
- Naghizadeh, M.; Torshizi MA, K.; Rahimi, S.; Engberg, R.M.; Dalgaard, T.S. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71.
- Manohar, P.; Tamhankar, A.J.; Lundborg, C.S.; Ramesh, N. Isolation, characterization and in vivo efficacy of Escherichia phage myPSH1131. PLoS ONE 2018, 13, e0206278.
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063.
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185.
- Miguéis, S.; Saraiva, C.; Esteves, A. Efficacy of LISTEX P100 at different concentrations for reduction of Listeria monocytogenes inoculated in sashimi. J. Food Prot. 2017, 80, 2094–2098.
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of bacteriophages in the agro-food sector: A long way toward approval. Front. Cell. Infect. Microbiol. 2018, 8, 296.
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565.
- Laforest, M.; Bisaillon, K.; Ciotola, M.; Cadieux, M.; Hébert, P.O.; Toussaint, V.; Svircev, A.M. Rapid identification of Erwinia amylovora and Pseudomonas syringae species and characterization of E. amylovora streptomycin resistance using quantitative PCR assays. Can. J. Microbiol. 2019, 65, 496–509.
- Hwang, M.S.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191.
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of bacteriophages against Pseudomonas syringae pv. actinidiae with potential use as natural antimicrobials in kiwifruit plants. Microorganisms 2020, 8, 974.
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage biocontrol to combat Pseudomonas syringae pathogens causing disease in cherry. Microb. Biotechnol. 2020, 13, 1428–1445.
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227.
- Czajkowski, R.; Smolarska, A.; Ozymko, Z. The viability of lytic bacteriophage ΦD5 in potato-associated environments and its effect on Dickeya solani in potato (Solanum tuberosum L.) plants. PLoS ONE 2017, 12, e0183200.
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Jacobs-Sera, D.; Hatfull, G.F.; Hansen, L.H. Unlocking the Potential of 46 New Bacteriophages for Biocontrol of Dickeya solani. Viruses 2018, 10, 621.
- Ye, M.; Sun, M.; Zhao, Y.; Jiao, W.; Xia, B.; Liu, M.; Feng, Y.; Zhang, Z.; Huang, D.; Huang, R.; et al. Targeted inactivation of antibiotic-resistant Escherichia coli and Pseudomonas aeruginosa in a soil-lettuce system by combined polyvalent bacteriophage and biochar treatment. Environ. Pollut. 2018, 241, 978–987.
- Kolenda, C.; Josse, J.; Medina, M.; Fevre, C.; Lustig, S.; Ferry, T.; Laurent, F. Evaluation of the activity of a combination of three bacteriophages alone or in association with antibiotics on Staphylococcus aureus embedded in biofilm or internalized in osteoblasts. Antimicrob. Agents Chemother. 2020, 64, e02231-19.
- Zhang, L.; Sun, L.; Wei, R.; Gao, Q.; He, T.; Xu, C.; Liu, X.; Wang, R. Intracellular Staphylococcus aureus control by virulent bacteriophages within MAC-T bovine mammary epithelial cells. Antimicrob. Agents Chemother. 2017, 61, e01990-16.
- Abedon, S.T. Spatial vulnerability: Bacterial arrangements, microcolonies, and biofilms as responses to low rather than high phage densities. Viruses 2012, 4, 663–687.
- Islam, M.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 2019, 11, 841.
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124.
- Grishin, A.V.; Karyagina, A.S.; Vasina, D.V.; Vasina, I.V.; Gushchin, V.A.; Lunin, V.G. Resistance to peptidoglycan-degrading enzymes. Crit. Rev. Microbiol. 2020, 46, 703–726.
- Schmelcher, M.; Powell, A.M.; Camp, M.J.; Pohl, C.S.; Donovan, D.M. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl. Microbiol. Biotechnol. 2015, 99, 8475–8486.
- Zhou, Y.; Zhang, H.; Bao, H.; Wang, X.; Wang, R. The lytic activity of recombinant phage lysin LysKΔamidase against staphylococcal strains associated with bovine and human infections in the Jiangsu province of China. Res. Vet. Sci. 2017, 111, 113–119.
- Anand, T.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Kumar, A.; Tripathi, B.N. Applications of Personalized Phage Therapy highlighting the importance of Bacteriophage Banks against Emerging Antimicrobial Resistance. Def. Life Sci. J. 2020, 5, 305–314.
- Kim, S.G.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; Jun, J.W.; et al. Synergistic phage–surfactant combination clears IgE-promoted Staphylococcus aureus aggregation in vitro and enhances the effect in vivo. Int. J. Antimicrob. Agents 2020, 56, 105997.
- Połaska, M.; Sokołowska, B. Review bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–347.
- Zulkarneev, E.R.; Aleshkin, A.V.; Kiseleva, I.A.; Rubalsky, E.O.; Rubalsky, O.V. Bacteriophage Cocktail Effectively Prolonging the Shelf-Life of Chilled Fish. Bull. Exp. Biol. Med. 2019, 167, 818–822.
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella bacteriophage, nisin and potassium sorbate and their combination on safety and shelf life of fresh chilled pork. Food Control 2017, 73, 869–877.
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39.
- Adesanya, O.; Oduselu, T.; Akin-Ajani, O.; Adewumi, O.M.; Ademowo, O.G. An exegesis of bacteriophage therapy: An emerging player in the fight against antimicrobial resistance. AIMS Microbiol. 2020, 6, 204–230.
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27.
- Hays, V.W. Benefits and risks of antibiotics use in agriculture. In Agricultural Uses of Antibiotics; Moats, W.A., Ed.; American Chemical Society: Washington, DC, USA, 1986; pp. 74–87.
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, e00066-18.
- Abedon, S.T.; García, P.; Mullany, P.; Aminov, R. Editorial: Phage therapy: Past, present and future. Front. Microbiol. 2017, 8.
- Tomat, D.D.; Migliore, L.; Aquili, V.; Quiberoni, A.D.L.; Balagué, C. Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli in meat products. Front. Cell. Infect. Microbiol. 2013, 3, 20.
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages reduce pathogenic Escherichia coli counts in mice without distorting gut microbiota. Front. Microbiol. 2019, 10, 1984.
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103.
- Cieplak, T.; Soffer, N.; Sulakvelidze, A.; Nielsen, D.S. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions 2018, while preserving a nontargeted representative commensal normal microbiota. Gut Microbes 2018, 9, 391–399.
- Kim, K.H.; Ingale, S.L.; Kim, J.S.; Lee, S.H.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage and probiotics both enhance the performance of growing pigs but bacteriophage are more effective. Anim. Feed. Sci. Technol. 2014, 196, 88–95.
- Kim, J.S.; Hosseindoust, A.; Lee, S.H.; Choi, Y.H.; Kim, M.J.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage cocktail and multistrain probiotics in the feed for weanling pigs: Effects on intestine morphology and targeted intestinal coliforms and Clostridium. Animal 2017, 11, 45–53.
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Noh, H.S.; Lee, J.H.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Dietary bacteriophages as an alternative for zinc oxide or organic acids to control diarrhea and improve the performance of weanling piglets. Vet. Med. 2017, 62, 53–61.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




