Bacteriophages, more commonly referred to as phages, are a class of viruses discovered for their bactericidal effects even before the discovery of penicillin and other antibiotics. An increasingly popular alternative to antibiotics is bacteriophages to control bacterial diseases. Their unique bactericidal properties make them an ideal alternative to antibiotics, as many countries begin to restrict the usage of antibiotics in agriculture. The recent evidence from within the past decade on the efficacy of phage therapy on common foodborne pathogens are analyzed, namely, Escherica coli, Staphylococcus aureus, Salmonella spp., and Campylobacter jejuni.
- antimicrobial resistance (AMR)
- Bacteriophages
- Agriculture
- food-borne pathogens
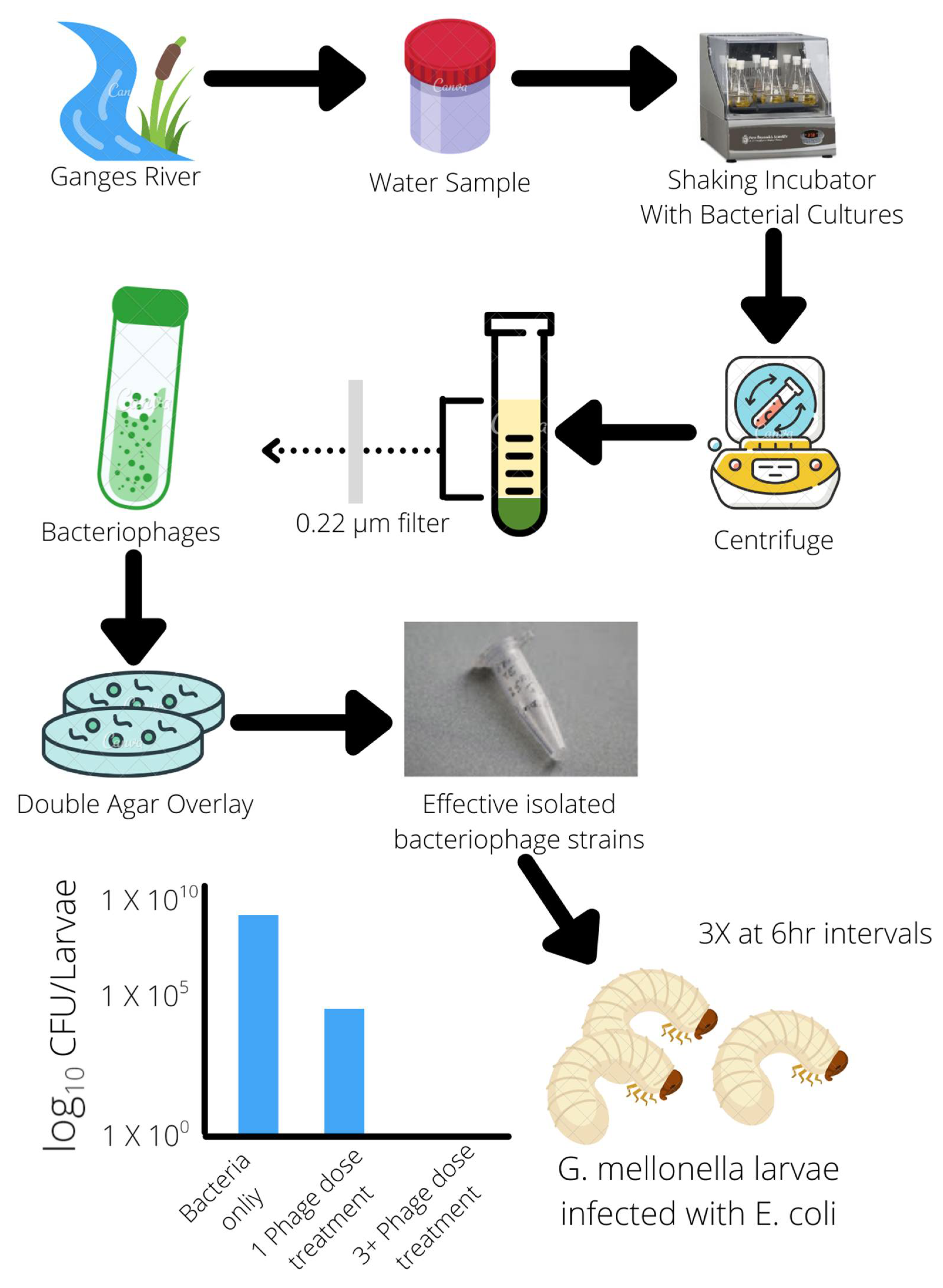
1. Bactericidal Effects of Bacteriophages Demonstrated in Agriculture
2. Advantages of Phage Therapy over Conventional Antibiotics
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10010046
References
- Capparelli, R.; Nocerino, N.; Iannaccone, M.; Ercolini, D.; Parlato, M.; Chiara, M.; Iannelli, D. Bacteriophage therapy of Salmonella enterica: A fresh appraisal of bacteriophage therapy. J. Infect. Dis. 2010, 201, 52–61.
- Cerveny, K.E.; DePaola, A.; Duckworth, D.H.; Gulig, P.A. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 2002, 70, 6251–6262.
- Capparelli, R.; Parlato, M.; Borriello, G.; Salvatore, P.; Iannelli, D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 2007, 51, 2765–2773.
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F.; et al. Development of a bacteriophage cocktail to constrain the emergence of phage-resistant Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 327.
- Wang, Z.; Zheng, P.; Ji, W.; Fu, Q.; Wang, H.; Yan, Y.; Sun, J. SLPW: A virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2016, 7, 934.
- Porter, J.; Anderson, J.; Carter, L.; Donjacour, E.; Paros, M. In vitro evaluation of a novel bacteriophage cocktail as a preventative for bovine coliform mastitis. J. Dairy Sci. 2016, 99, 2053–2062.
- Yulinery, T.; Triana, E.; Suharna, N.; Nurhidayat, N. Isolation and anti-Escherichia coli biofilm activity of lytic bacteriophages isolated from water environment in vitro. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2019; Volume 308, p. 012010.
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell. Infect. Microbiol. 2021, 11, 555.
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023.
- Naghizadeh, M.; Torshizi MA, K.; Rahimi, S.; Engberg, R.M.; Dalgaard, T.S. Effect of serum anti-phage activity on colibacillosis control by repeated phage therapy in broilers. Vet. Microbiol. 2019, 234, 61–71.
- Manohar, P.; Tamhankar, A.J.; Lundborg, C.S.; Ramesh, N. Isolation, characterization and in vivo efficacy of Escherichia phage myPSH1131. PLoS ONE 2018, 13, e0206278.
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.C.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063.
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185.
- Miguéis, S.; Saraiva, C.; Esteves, A. Efficacy of LISTEX P100 at different concentrations for reduction of Listeria monocytogenes inoculated in sashimi. J. Food Prot. 2017, 80, 2094–2098.
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of bacteriophages in the agro-food sector: A long way toward approval. Front. Cell. Infect. Microbiol. 2018, 8, 296.
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565.
- Laforest, M.; Bisaillon, K.; Ciotola, M.; Cadieux, M.; Hébert, P.O.; Toussaint, V.; Svircev, A.M. Rapid identification of Erwinia amylovora and Pseudomonas syringae species and characterization of E. amylovora streptomycin resistance using quantitative PCR assays. Can. J. Microbiol. 2019, 65, 496–509.
- Hwang, M.S.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191.
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of bacteriophages against Pseudomonas syringae pv. actinidiae with potential use as natural antimicrobials in kiwifruit plants. Microorganisms 2020, 8, 974.
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage biocontrol to combat Pseudomonas syringae pathogens causing disease in cherry. Microb. Biotechnol. 2020, 13, 1428–1445.
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227.
- Czajkowski, R.; Smolarska, A.; Ozymko, Z. The viability of lytic bacteriophage ΦD5 in potato-associated environments and its effect on Dickeya solani in potato (Solanum tuberosum L.) plants. PLoS ONE 2017, 12, e0183200.
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Jacobs-Sera, D.; Hatfull, G.F.; Hansen, L.H. Unlocking the Potential of 46 New Bacteriophages for Biocontrol of Dickeya solani. Viruses 2018, 10, 621.
- Ye, M.; Sun, M.; Zhao, Y.; Jiao, W.; Xia, B.; Liu, M.; Feng, Y.; Zhang, Z.; Huang, D.; Huang, R.; et al. Targeted inactivation of antibiotic-resistant Escherichia coli and Pseudomonas aeruginosa in a soil-lettuce system by combined polyvalent bacteriophage and biochar treatment. Environ. Pollut. 2018, 241, 978–987.
- Kolenda, C.; Josse, J.; Medina, M.; Fevre, C.; Lustig, S.; Ferry, T.; Laurent, F. Evaluation of the activity of a combination of three bacteriophages alone or in association with antibiotics on Staphylococcus aureus embedded in biofilm or internalized in osteoblasts. Antimicrob. Agents Chemother. 2020, 64, e02231-19.
- Zhang, L.; Sun, L.; Wei, R.; Gao, Q.; He, T.; Xu, C.; Liu, X.; Wang, R. Intracellular Staphylococcus aureus control by virulent bacteriophages within MAC-T bovine mammary epithelial cells. Antimicrob. Agents Chemother. 2017, 61, e01990-16.
- Abedon, S.T. Spatial vulnerability: Bacterial arrangements, microcolonies, and biofilms as responses to low rather than high phage densities. Viruses 2012, 4, 663–687.
- Islam, M.; Zhou, Y.; Liang, L.; Nime, I.; Liu, K.; Yan, T.; Wang, X.; Li, J. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 2019, 11, 841.
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124.
- Grishin, A.V.; Karyagina, A.S.; Vasina, D.V.; Vasina, I.V.; Gushchin, V.A.; Lunin, V.G. Resistance to peptidoglycan-degrading enzymes. Crit. Rev. Microbiol. 2020, 46, 703–726.
- Schmelcher, M.; Powell, A.M.; Camp, M.J.; Pohl, C.S.; Donovan, D.M. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl. Microbiol. Biotechnol. 2015, 99, 8475–8486.
- Zhou, Y.; Zhang, H.; Bao, H.; Wang, X.; Wang, R. The lytic activity of recombinant phage lysin LysKΔamidase against staphylococcal strains associated with bovine and human infections in the Jiangsu province of China. Res. Vet. Sci. 2017, 111, 113–119.
- Anand, T.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Kumar, A.; Tripathi, B.N. Applications of Personalized Phage Therapy highlighting the importance of Bacteriophage Banks against Emerging Antimicrobial Resistance. Def. Life Sci. J. 2020, 5, 305–314.
- Kim, S.G.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; Jun, J.W.; et al. Synergistic phage–surfactant combination clears IgE-promoted Staphylococcus aureus aggregation in vitro and enhances the effect in vivo. Int. J. Antimicrob. Agents 2020, 56, 105997.
- Połaska, M.; Sokołowska, B. Review bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–347.
- Zulkarneev, E.R.; Aleshkin, A.V.; Kiseleva, I.A.; Rubalsky, E.O.; Rubalsky, O.V. Bacteriophage Cocktail Effectively Prolonging the Shelf-Life of Chilled Fish. Bull. Exp. Biol. Med. 2019, 167, 818–822.
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella bacteriophage, nisin and potassium sorbate and their combination on safety and shelf life of fresh chilled pork. Food Control 2017, 73, 869–877.
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39.
- Adesanya, O.; Oduselu, T.; Akin-Ajani, O.; Adewumi, O.M.; Ademowo, O.G. An exegesis of bacteriophage therapy: An emerging player in the fight against antimicrobial resistance. AIMS Microbiol. 2020, 6, 204–230.
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27.
- Hays, V.W. Benefits and risks of antibiotics use in agriculture. In Agricultural Uses of Antibiotics; Moats, W.A., Ed.; American Chemical Society: Washington, DC, USA, 1986; pp. 74–87.
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, e00066-18.
- Abedon, S.T.; García, P.; Mullany, P.; Aminov, R. Editorial: Phage therapy: Past, present and future. Front. Microbiol. 2017, 8.
- Tomat, D.D.; Migliore, L.; Aquili, V.; Quiberoni, A.D.L.; Balagué, C. Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli in meat products. Front. Cell. Infect. Microbiol. 2013, 3, 20.
- Dissanayake, U.; Ukhanova, M.; Moye, Z.D.; Sulakvelidze, A.; Mai, V. Bacteriophages reduce pathogenic Escherichia coli counts in mice without distorting gut microbiota. Front. Microbiol. 2019, 10, 1984.
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103.
- Cieplak, T.; Soffer, N.; Sulakvelidze, A.; Nielsen, D.S. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions 2018, while preserving a nontargeted representative commensal normal microbiota. Gut Microbes 2018, 9, 391–399.
- Kim, K.H.; Ingale, S.L.; Kim, J.S.; Lee, S.H.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage and probiotics both enhance the performance of growing pigs but bacteriophage are more effective. Anim. Feed. Sci. Technol. 2014, 196, 88–95.
- Kim, J.S.; Hosseindoust, A.; Lee, S.H.; Choi, Y.H.; Kim, M.J.; Lee, J.H.; Kwon, I.K.; Chae, B.J. Bacteriophage cocktail and multistrain probiotics in the feed for weanling pigs: Effects on intestine morphology and targeted intestinal coliforms and Clostridium. Animal 2017, 11, 45–53.
- Hosseindoust, A.R.; Lee, S.H.; Kim, J.S.; Choi, Y.H.; Noh, H.S.; Lee, J.H.; Jha, P.K.; Kwon, I.K.; Chae, B.J. Dietary bacteriophages as an alternative for zinc oxide or organic acids to control diarrhea and improve the performance of weanling piglets. Vet. Med. 2017, 62, 53–61.
