Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgio Ciprandi | -- | 2002 | 2023-07-17 14:49:53 | | | |
| 2 | Rita Xu | Meta information modification | 2002 | 2023-07-18 03:51:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciprandi, G.; Varriccchio, A. Sobrerol. Encyclopedia. Available online: https://encyclopedia.pub/entry/46874 (accessed on 11 March 2026).
Ciprandi G, Varriccchio A. Sobrerol. Encyclopedia. Available at: https://encyclopedia.pub/entry/46874. Accessed March 11, 2026.
Ciprandi, Giorgio, Attilio Varriccchio. "Sobrerol" Encyclopedia, https://encyclopedia.pub/entry/46874 (accessed March 11, 2026).
Ciprandi, G., & Varriccchio, A. (2023, July 17). Sobrerol. In Encyclopedia. https://encyclopedia.pub/entry/46874
Ciprandi, Giorgio and Attilio Varriccchio. "Sobrerol." Encyclopedia. Web. 17 July, 2023.
Copy Citation
Respiratory tract infections (RTIs) are usually characterized by mucus hypersecretion. This condition may worsen and prolong symptoms and signs. For this reason, reducing mucus production and improving mucus removal represent relevant aspects of managing patients with RTIs. In this regard, mucoactive drugs may be effective. Mucoactive agents constitute a large class of compounds characterized by different mechanisms of action. Sobrerol is a monoterpene able to fluidify mucus, increase mucociliary clearance, and exert antioxidant activity. Sobrerol is available in various formulations (granules, syrup, nebulized, and suppository). Sobrerol has been on the market for over 50 years.
mucus
sobrerol
mucolytic agent
respiratory tract infections
1. Background on Respiratory Tract Infections
Respiratory tract infections (RTIs) are widespread diseases and constitute among the most frequent causes of access to primary care doctors, as recently reported by a systematic review including data from 12 countries across five continents [1]. Acute upper RTIs are prevalent and usually recognize a bacterial or viral cause, although viral forms are the most common, as reported in the literature [2]. It has to be underlined that, in clinical practice, the diagnosis is given without a known pathogen. Many viral agents may cause acute upper RTIs, but rhinovirus, coronavirus, syncytial, influenza, parainfluenza, adenovirus, coxsackievirus, echovirus, paramyxovirus, and enterovirus are the most common [3][4][5][6][7][8]. Viral respiratory infections are typically seasonal, such as increased infectivity during cold seasons [9]. Exposure to cold exerts different mechanisms to promote RTIs, including impaired mucociliary clearance, deficient nasal defense, and reduced immune function [10]. Clinically, researchers have to consider that all respiratory viruses may induce an influenza-like illness (ILI), also named flu-like syndrome [11]. Indeed, ILI represents a typical acute viral illness and mimics the clinical features of influenza [12]. ILI is also an acute medical condition characterized by general and respiratory symptoms. In particular, the definition of ILI (common throughout Europe) includes “any person who presents a sudden and rapid onset of at least one of the following general symptoms: fever or feverishness, malaise/exhaustion, headache, myalgia, and at least one of the following respiratory symptoms: cough, sore throat, and wheezing” [13]. However, if milder manifestations occur, the typical common cold has to be considered [14]. Even if AURI may be associated with lower respiratory tract involvement, a healthy and immunocompetent subject usually presents only symptoms concerning the upper respiratory tract.
2. Practical Management
From a clinical point of view, AURI management is generally grounded in a quick treatment that presumes a diagnosis usually based on clinical and epidemiological criteria [15]. The most common symptoms include sneezing, rhinorrhea, nasal congestion, hypo/anosmia, hypo/ageusia, facial pressure, sore throat, cough, headache, discomfort, myalgias, and low-grade fever [16]. Notably, these symptoms usually last less than ten days, apart from the cough, which tends to last longer [17]. The treatment should be timely and appropriate for every single patient. However, if symptoms persist longer or worsen, a trivial common cold may evolve into rhinosinusitis, needing an appropriate work-up [18]. As mentioned above, it has to be underlined that the cough may even last for more than a month in some subjects [19]. Another aspect that has to be considered is that even if these symptoms are self-resolving, they are still particularly annoying. The parents want to solve them immediately, mainly if a fever is present. Fever often instills fear in parents, even unmotivated fear, so a real fever-phobia is generated [20].
Consequently, doctors prescribe symptomatic relievers as a first-line treatment [21]. The main goal of treatment is, in fact, the prompt reduction of symptom intensity and duration. It is vital to recommend to patients and parents that antibiotics should not be used unless a bacterial complication occurs [22]. Non-steroidal anti-inflammatory drugs (NSAIDs), nasal lavage, and non-pharmacologic remedies are usually sufficient to rapidly cure the most acute viral infections [23]. Another aspect that researchers still need to consider has been further emphasized by the recent COVID-19 pandemic [24]. Researchers should believe that facing an infection is always accompanied by inflammation [25]. As a result, inflammation dampening represents a leading therapeutic target in managing infections. The second lesson provided by COVID-19 infection concerns the dramatic outbreak of other RTIs that occurred successively. The explanation of this infections epidemy depends on the restrictive measure (lockdown, mask use, and social distancing) that significantly diminished the incidence of RTIs [26]. The paradigmatic example was the negligible prevalence of bronchiolitis during the early COVID-19 pandemic [27]. However, since the slackening of restrictive measures, there has been a surge in cases of bronchiolitis that has put a strain on the pediatric hospital network [28]. The 2022/2023 seasonal influenza epidemic had an early onset, extraordinary incidence, clinical severity, and persistent duration of the epidemic plateau [29].
3. The Relevance of Mucus Hyperproduction in Respiratory Tract Infections
The respiratory mucus secretion is a complex mixture produced by different structures, including submucosal glands and secreting epithelial cells (goblet cells and Clara cells) [30][31]. The submucosal glands are tubular/tubulacinar formations producing mucous, serous, or seromucous secretions. The goblets cells are mucous-secreting cells intercalated in the cylindrical ciliated epithelium lining, disappearing in the terminal bronchioles. The Clara cells are intercalated with the low or cubic cylindrical epithelium, ciliated or not, which extends in a single layer along the peripheral airways. These cells produce both a mucous secretion (like goblet cells) and a lipoprotein secretion (like type B pneumocytes), which can be identified as an alveolar surfactant [32][33].
The amount of mucus produced depends on the number of mucus-secreting cells present at that level, which in turn is related to the total surface area of the airways; thus, mucus production occurs more in the peripheral airways than in the central airways [34]. Physiologically, an adult produces about 10–100 mL of mucus per day, and the amount of mucus that reaches the trachea is approximately 10–20 mL/day [30][31]. The superficial layer constitutes the ‘sol’ phase of the mucus, very rich in water, about 3 microns thick, and occupies almost the entire length of the cilia of the epithelial cells [35]. Above, there is a dense layer: the ‘gel’ phase. It mainly contains glycoproteins (mucins), characterized by a central protein structure anchored to lateral polysaccharide chains formed by sialic acid (sialomucins) or fucose (fucosomucins). In the gel layer, molecules with anti-infective activity exist, such as secretory IgA (S-IgA), lactoferrin, and lysozyme. In addition, it has been hypothesized that a third layer consisting of surfactant exists between the sol and gel layers [31]. Usually, only the gel layer is transported, but the sol layer seems essential for mucus transport because it allows the cilia to beat effectively [33]. Mucus transport is governed by the mechanical forces of ciliary beating and airflow, counteracted by the friction and inertia of the mucus itself [36]. Mucociliary clearance is prevalent in the peripheral airways. Each ciliary cell has about 200 cilia, and the cilia are equipped with ‘claws’ that reach into the gel layer and push it toward the oropharynx [34][35]. The mucociliary clearance efficacy depends on the airflow velocity, a function of the airway diameter, and the pressure the expiratory muscles create [37]. Moreover, in the first years of life, the small airways tend to collapse during normal breathing, and the ciliary machinery develops progressively [38].
4. Sobrerol
Sobrerol has been on the market in many European countries for over 50 years since its launch in the early 1970s. Sobrerol (5-hydroxy-α, α, 4-trimethyl-3-cyclohexene-1-methanol) is a monocyclic monoterpene with two hydroxyl functions. Various effects characterize it (Figure 1). Researchers focused on sobrerol as it is commonly used, and a large body of evidence exists on this compound. However, most studies are old, and researchers would propose new possible strategies for their use.
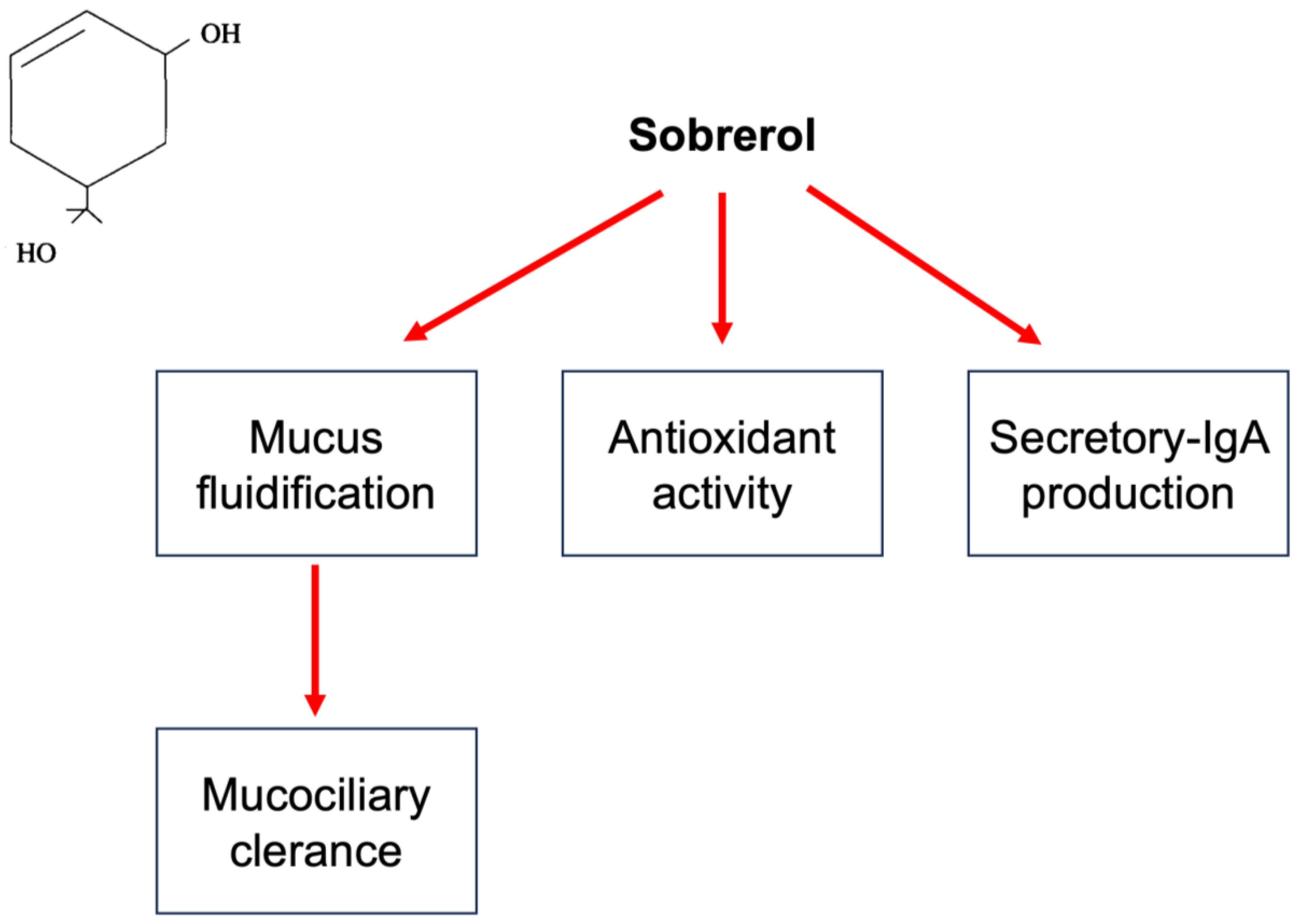
Figure 1. Mechanisms of action of sobrerol. In the left quadrant is reported the chemical molecule. Sobrerol, as schematized, induces mucus fluidification and consequently improves mucociliary clearance, exerts antioxidant activity, and increases sIgA production.
Sobrerol in vivo increased mucus production and ciliary motility, thus improving mucociliary clearance [39]. In addition, sobrerol reduced the viscosity of tracheobronchial mucus without causing any alterations of the alveolar surfactants [40]. Radical scavenging activities have also been reported [41]. Finally, sobrerol may increase the production of secretory IgAs. Sobrerol is available in different formulations, including syrup, water-soluble sachets, nebulization, intramuscular (or intravenous) vials, and suppositories. The main indication is the treatment of respiratory disorders characterized by thick, viscous hypersecretion. The recommended oral dose in adults is 600 mg (equivalent to the contents of two sachets) per day for up to three days. In children, this dosage is halved. The dose administered by the aerosol route is one vial for nebulization, containing 40 mg of sobrerol, one to two times daily. Clinical studies in the literature have used a treatment duration of up to three months or a maximum daily dosage of 900 mg for ten consecutive days [39]. The only contraindication is used in children under 30 months of age or with a history of epilepsy or febrile convulsions, as well as hypersensitivity to the active ingredient or any excipients used in the various formulations. Particular precaution must, however, be observed in subjects with severe respiratory insufficiency, asthmatics, and debilitated patients, as the increased fluidity of secretions requires effective expectoration.
The first pediatric RCT study was performed in 1981. This RCT study was conducted as double-blind, randomized, and placebo-controlled and had 100 patients aged between 12 and 74 years with acute or chronic upper or lower respiratory tract infections [42]. Sobrerol syrup (260 mg) was administered with carbocysteine capsules (375 mg) four times daily for 21 days. This combination significantly improved objective and subjective clinical parameters and lung function compared to a placebo. In addition, the treatment was well tolerated. Unfortunately, pediatric outcomes could not be deduced. The second study evaluated the efficacy and safety of oral sobrerol compared to oral N-acetylcysteine in 40 children with acute respiratory diseases (bronchitis or pharyngo-tracheobronchitis) [43]. Sobrerol was administered as granules in one-dose sachets for three days at 100 mg/3 times a day. N-acetylcysteine granules were used at 300 mg/day for three days. Clinical parameters and biological data, including rheological examination of expectorate, were considered at baseline and the end of the course. The two treatments were effective without significant differences. However, sobrerol induced a better reduction of expectorating viscosity and was better tolerated than N-acetylcysteine. A third RCT study was conducted as double-blind, randomized, and placebo-controlled in 30 children with pertussis aged between 10 months and 12 years [44]. The measured outcomes included clinical and functional parameters. The treatment consisted of an oral combination of clofedanol (central antitussive agent) 1.62 mg/Kg/daily with sobrerol 3.6 mg/Kg/day for 15 days. The active treatment was safe and significantly improved signs and lung function compared to placebo. The first open study included 40 children under five years old with acute and recurrent bronchitis [45]. This study randomly compared oral sobrerol (50–100 mg/twice daily) with oral bromhexine (2–4 mg/three times daily) for two weeks. The outcome was an improvement rate. The results showed that both drugs were effective, but no significant differences occurred. The second open study retrospectively recruited 59 children with acute upper RTIs and wet cough aged between 3 and 14 years [46]. This study compared children treated with oral antibiotics (amoxicillin or a macrolide) with children treated with nebulized mucoactive drugs (sobrerol or N-acetylcysteine) used in standard practice. The children treated with mucolytics significantly improved clinical parameters compared with children treated with antibiotics. A third open study considered a group of 30 children (5–10 years old) with secretory otitis media and treated with nebulized sobrerol (one 40 mg vial/day) alone for ten consecutive days [47]. The treatment improved clinical outcomes (nasal obstruction, deafness, and earache) and impedance values. The improvement depended on mucus fluidification in the upper airways. The previous observational study included 20 children (6 months–2 years old) with pertussis [48]. The primary outcome was the time course to symptom resolution. Children were treated with oral or rectal sobrerol (four times daily), oral salbutamol, and oral erythromycin (40 mg/kg/day) until clinical resolution. This treatment was considered better than historical controls (antibiotics alone or associated with hyperimmune gamma globulins and/or cough sedatives).
Notably, all these studies were conducted in Italy. The global outcomes were positive; however, the records were heterogeneous concerning the medical conditions and treatments. The clinical judgment was always favorable, as confirmed by the consolidated use in clinical practice. However, regulatory agencies recently restricted the treatment duration to only three days for all formulations. This limitation may prompt new possible strategies for using sobrerol.
References
- Finley, C.R.; Chan, D.S.; Garrison, S.; Korownyk, C.; Kolber, M.R.; Campbell, S. What are the most common conditions in primary care? Systematic review. Can. Fam. Physician 2018, 64, 832–840.
- Murgia, V.; Manti, S.; Licari, A.; De Filippo, M.; Ciprandi, G.; Marseglia, G.L. Upper Respiratory Tract Infection-Associated Acute Cough and the Urge to Cough: New Insights for Clinical Practice. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 3–11.
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141.
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432.
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810.
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554.
- Del Vecchio, A.; Ferrara, T.; Maglione, M.; Capasso, L.; Raimondi, F. New perspectives in Respiratory Syncitial Virus infection. J. Matern. Fetal Neonatal Med. 2013, 26 (Suppl. S2), 55–59.
- Schuster, J.E.; Williams, J.V. Emerging Respiratory Viruses in Children. Infect. Dis. Clin. N. Am. 2018, 32, 65–74.
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101.
- Eccles, R.; Wilkinson, J.E. Exposure to cold and acute upper respiratory tract infection. Rhinology 2015, 53, 99–106.
- Humiston, S.G.; Pham, T.N. Influenza-Like Illness Diagnosis and Management in the Acute Care Setting. Pediatr. Emerg. Care 2016, 32, 875–882.
- Spencer, J.A.; Shutt, D.P.; Moser, S.K.; Clegg, H.; Wearing, H.J.; Mukundan, H.; Manore, C.A. Distinguishing viruses responsible for influenza-like illness. J. Theor. Biol. 2022, 545, 111145.
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Health Organ. 2018, 96, 122–128.
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59.
- Passioti, M.; Maggina, P.; Megremis, S.; Papadopoulos, N.G. The common cold: Potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014, 14, 413.
- Centers for Disease Control and Prevention. Common Cold. Available online: https://www.cdc.gov/dotw/common-cold/index.html (accessed on 8 July 2023).
- DeGeorge, K.C.; Ring, D.J.; Dalrymple, S.N. Treatment of the common cold. Am. Fam. Physician 2019, 100, 281–289.
- Fokkens, W.; Lund, V.; Hopkins, C.; Hellings, P.; Kern, R.; Reitsma, S.; Toppila-salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464.
- Fashner, J.; Ericson, K.; Werner, S. Treatment of the common cold in children and adults. Am Fam Physician 2012, 86, 153–159.
- Chiappini, E.; Parretti, A.; Becherucci, P.; Pierattelli, M.; Bonsignori, F.; Galli, L.; de Martino, M. Parental and medical knowledge and management of fever in Italian pre-school children. BMC Pediatr. 2012, 12, 97.
- Ciprandi, G.; Tosca, M.A. Non-pharmacological remedies for post-viral acute cough. Monaldi Arch. Chest Dis. 2021, 92.
- Centers for Disease Control and Prevention. Antibiotic Use in the United States, 2023: Progress and Opportunities. Available online: https://www.cdc.gov/antibiotic-use/pdfs/stewardship-report-2021-H.pdf (accessed on 22 June 2023).
- Jaume, F.; Valls-Mateus, M.; Mullol, J. Common Cold and Acute Rhinosinusitis: Up-to-Date Management in 2020. Curr. Allergy Asthma Rep. 2020, 20, 28.
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051.
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998.
- Noureddine, F.Y.; Chakkour, M.; El Roz, A.; Reda, J.; Al Sahily, R.; Assi, A.; Joma, M.; Salami, H.; Ghssein, G. The emergence of SARS-CoV-2 variant(s) and its impact on the prevalence of COVID-19 cases in the Nabatieh Region, Lebanon. Med. Sci. 2021, 9, 40.
- Van Brusselen, D.; De Troeyer, K.; Ter Haar, E.; Vander Auwera, A.; Poschet, K.; Van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; Van Herendael, B.; et al. Bronchiolitis in COVID-19 times: A nearly absent disease? Eur. J. Pediatr. 2021, 180, 1969–1973.
- Cardenas, J.; Pringle, C.; Filipp, S.L.; Gurka, M.J.; Ryan, K.A.; Avery, K.L. Changes in Critical Bronchiolitis After COVID-19 Lockdown. Cureus 2022, 14, e25064.
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2365.
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247.
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176.
- Kumari, C.; Gupta, R.; Sharma, M.; Jacob, J.; Narayan, R.K.; Sahni, D.; Kumar, A. Morpho-functional characterization of the submucosal glands at the nasopharyngeal end of the auditory tube in humans. J. Anat. 2023, 242, 771–780.
- Birchenough, G.M.; Johansson, M.E.V.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719.
- Van der Schans, C.P. Bronchial mucus transport. Respir. Care 2007, 52, 1150–1156.
- Widdicombe, J.H. Regulation of the depth and composition of airways surface liquid. J. Anat. 2002, 201, 313–318.
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15.
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220.
- Francis, R.J.; Chatterjee, B.; Loges, N.T.; Zentgraf, H.; Omran, H.; Lo, C.W. Initiation and maturation of cilia-generated flow in newborn and postnatal mouse airway. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, 1067–1075.
- Scaglione, F.; Petrini, O. Mucoactive Agents in the Therapy of Upper Respiratory Airways Infections: Fair to Describe Them Just as Mucoactive? Clin. Med. Insights Ear Nose Throat 2019, 12, 1–9.
- Rogers, D.F. Mucoactive agents for airway mucus hypersecretory diseases. Respir. Care 2007, 52, 1176–1193.
- Braga, P.C.; Culici, M.; Dal Sasso, M.; Falch, M.; Spallino, A. Antiradical activity of sobrerol investigated by electron paramagnetic resonance (EPR). Giorn. It Mal. Tor. 2009, 63, 263–267.
- Milvio, C.; Di Tommaso, G.; Mader, R. Traitement des hypersécretions bronchiques dans les bronchopneumopathies aiguës et chroniques—Étude contrôlée d’un nouveau composé à action mucolytique. Acta Ther. 1981, 7, 243–260.
- Seidita, F.; Deiana, M.; Careddu, P. Acute bronchial diseases in paediatrics: Therapeutic approach with sobrerol granules. G. Ital. Mal. Torace 1984, 38, 191–194.
- Miraglia del Giudice, M.; Capristo, A.F.; Mirra, G.; Maiello, N.; Coppola, T. Controlled double-blind study on the efficacy of clofedanol-sobrerol in the treatment of pediatric pertussis. Minerva Pediatr. 1984, 36, 1199–1206.
- Azzollini, E.; Bosi, M.; Mantegazza, M.; Piceci, E.; Careddu, P. Sobrerol (Sobrepim) administered dropwise to children with acute hypersecretory bronchopulmonary disease—A controlled trial v bromhexine. Clin. Trials J. 1990, 27, 241–249.
- Zanasi, A.; Cazzato, S.; Aprile, A.; Mazzolini, M.; Zenezini, C.; Pandolfi, P. Are antibiotics effective in treating children with acute moist cough? a retrospective study vs symptomatic therapy. Multidiscip. Respir. Med. 2012, 7, 1–5.
- Bellussi, L.; Bernocchi, D.; Ciferri, G.; Manini, G.; Passali, D. Sobrerol in the treatment of secretory otitis media in childhood. J. Int. Med. Res. 1989, 17, 277–286.
- Crosca, V.; Ajello, A.; Crosca, C.; Minniti, A. Salbutamol combined with erythromycin and sobrerol in the therapy of pertussis. Arch. Sci. Med. 1982, 139, 247–250.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
18 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





