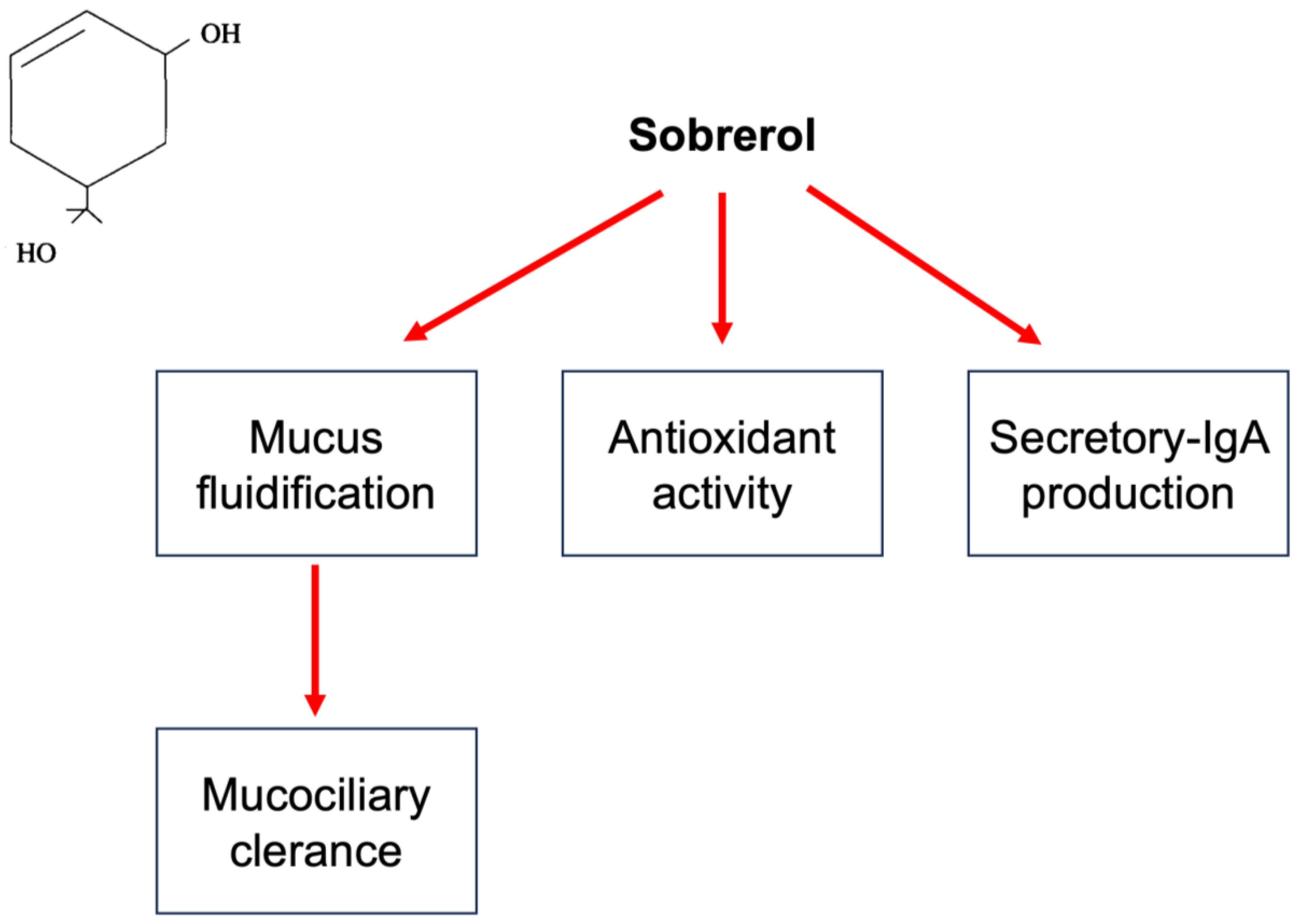
Respiratory tract infections (RTIs) are usually characterized by mucus hypersecretion. This condition may worsen and prolong symptoms and signs. For this reason, reducing mucus production and improving mucus removal represent relevant aspects of managing patients with RTIs. In this regard, mucoactive drugs may be effective. Mucoactive agents constitute a large class of compounds characterized by different mechanisms of action. Sobrerol is a monoterpene able to fluidify mucus, increase mucociliary clearance, and exert antioxidant activity. Sobrerol is available in various formulations (granules, syrup, nebulized, and suppository). Sobrerol has been on the market for over 50 years.
- mucus
- sobrerol
- mucolytic agent
- respiratory tract infections
1. Background on Respiratory Tract Infections
2. Practical Management
3. The Relevance of Mucus Hyperproduction in Respiratory Tract Infections
4. Sobrerol
This entry is adapted from the peer-reviewed paper 10.3390/children10071210
References
- Finley, C.R.; Chan, D.S.; Garrison, S.; Korownyk, C.; Kolber, M.R.; Campbell, S. What are the most common conditions in primary care? Systematic review. Can. Fam. Physician 2018, 64, 832–840.
- Murgia, V.; Manti, S.; Licari, A.; De Filippo, M.; Ciprandi, G.; Marseglia, G.L. Upper Respiratory Tract Infection-Associated Acute Cough and the Urge to Cough: New Insights for Clinical Practice. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 3–11.
- Esneau, C.; Duff, A.C.; Bartlett, N.W. Understanding Rhinovirus Circulation and Impact on Illness. Viruses 2022, 14, 141.
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432.
- Hutchinson, E.C. Influenza Virus. Trends Microbiol. 2018, 26, 809–810.
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554.
- Del Vecchio, A.; Ferrara, T.; Maglione, M.; Capasso, L.; Raimondi, F. New perspectives in Respiratory Syncitial Virus infection. J. Matern. Fetal Neonatal Med. 2013, 26 (Suppl. S2), 55–59.
- Schuster, J.E.; Williams, J.V. Emerging Respiratory Viruses in Children. Infect. Dis. Clin. N. Am. 2018, 32, 65–74.
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101.
- Eccles, R.; Wilkinson, J.E. Exposure to cold and acute upper respiratory tract infection. Rhinology 2015, 53, 99–106.
- Humiston, S.G.; Pham, T.N. Influenza-Like Illness Diagnosis and Management in the Acute Care Setting. Pediatr. Emerg. Care 2016, 32, 875–882.
- Spencer, J.A.; Shutt, D.P.; Moser, S.K.; Clegg, H.; Wearing, H.J.; Mukundan, H.; Manore, C.A. Distinguishing viruses responsible for influenza-like illness. J. Theor. Biol. 2022, 545, 111145.
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Health Organ. 2018, 96, 122–128.
- Heikkinen, T.; Järvinen, A. The common cold. Lancet 2003, 361, 51–59.
- Passioti, M.; Maggina, P.; Megremis, S.; Papadopoulos, N.G. The common cold: Potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014, 14, 413.
- Centers for Disease Control and Prevention. Common Cold. Available online: https://www.cdc.gov/dotw/common-cold/index.html (accessed on 8 July 2023).
- DeGeorge, K.C.; Ring, D.J.; Dalrymple, S.N. Treatment of the common cold. Am. Fam. Physician 2019, 100, 281–289.
- Fokkens, W.; Lund, V.; Hopkins, C.; Hellings, P.; Kern, R.; Reitsma, S.; Toppila-salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464.
- Fashner, J.; Ericson, K.; Werner, S. Treatment of the common cold in children and adults. Am Fam Physician 2012, 86, 153–159.
- Chiappini, E.; Parretti, A.; Becherucci, P.; Pierattelli, M.; Bonsignori, F.; Galli, L.; de Martino, M. Parental and medical knowledge and management of fever in Italian pre-school children. BMC Pediatr. 2012, 12, 97.
- Ciprandi, G.; Tosca, M.A. Non-pharmacological remedies for post-viral acute cough. Monaldi Arch. Chest Dis. 2021, 92.
- Centers for Disease Control and Prevention. Antibiotic Use in the United States, 2023: Progress and Opportunities. Available online: https://www.cdc.gov/antibiotic-use/pdfs/stewardship-report-2021-H.pdf (accessed on 22 June 2023).
- Jaume, F.; Valls-Mateus, M.; Mullol, J. Common Cold and Acute Rhinosinusitis: Up-to-Date Management in 2020. Curr. Allergy Asthma Rep. 2020, 20, 28.
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051.
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998.
- Noureddine, F.Y.; Chakkour, M.; El Roz, A.; Reda, J.; Al Sahily, R.; Assi, A.; Joma, M.; Salami, H.; Ghssein, G. The emergence of SARS-CoV-2 variant(s) and its impact on the prevalence of COVID-19 cases in the Nabatieh Region, Lebanon. Med. Sci. 2021, 9, 40.
- Van Brusselen, D.; De Troeyer, K.; Ter Haar, E.; Vander Auwera, A.; Poschet, K.; Van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; Van Herendael, B.; et al. Bronchiolitis in COVID-19 times: A nearly absent disease? Eur. J. Pediatr. 2021, 180, 1969–1973.
- Cardenas, J.; Pringle, C.; Filipp, S.L.; Gurka, M.J.; Ryan, K.A.; Avery, K.L. Changes in Critical Bronchiolitis After COVID-19 Lockdown. Cureus 2022, 14, e25064.
- Krumbein, H.; Kümmel, L.S.; Fragkou, P.C.; Thölken, C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Renz, H.; Skevaki, C. Respiratory viral co-infections in patients with COVID-19 and associated outcomes: A systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2365.
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247.
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176.
- Kumari, C.; Gupta, R.; Sharma, M.; Jacob, J.; Narayan, R.K.; Sahni, D.; Kumar, A. Morpho-functional characterization of the submucosal glands at the nasopharyngeal end of the auditory tube in humans. J. Anat. 2023, 242, 771–780.
- Birchenough, G.M.; Johansson, M.E.V.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719.
- Van der Schans, C.P. Bronchial mucus transport. Respir. Care 2007, 52, 1150–1156.
- Widdicombe, J.H. Regulation of the depth and composition of airways surface liquid. J. Anat. 2002, 201, 313–318.
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15.
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220.
- Francis, R.J.; Chatterjee, B.; Loges, N.T.; Zentgraf, H.; Omran, H.; Lo, C.W. Initiation and maturation of cilia-generated flow in newborn and postnatal mouse airway. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, 1067–1075.
- Scaglione, F.; Petrini, O. Mucoactive Agents in the Therapy of Upper Respiratory Airways Infections: Fair to Describe Them Just as Mucoactive? Clin. Med. Insights Ear Nose Throat 2019, 12, 1–9.
- Rogers, D.F. Mucoactive agents for airway mucus hypersecretory diseases. Respir. Care 2007, 52, 1176–1193.
- Braga, P.C.; Culici, M.; Dal Sasso, M.; Falch, M.; Spallino, A. Antiradical activity of sobrerol investigated by electron paramagnetic resonance (EPR). Giorn. It Mal. Tor. 2009, 63, 263–267.
- Milvio, C.; Di Tommaso, G.; Mader, R. Traitement des hypersécretions bronchiques dans les bronchopneumopathies aiguës et chroniques—Étude contrôlée d’un nouveau composé à action mucolytique. Acta Ther. 1981, 7, 243–260.
- Seidita, F.; Deiana, M.; Careddu, P. Acute bronchial diseases in paediatrics: Therapeutic approach with sobrerol granules. G. Ital. Mal. Torace 1984, 38, 191–194.
- Miraglia del Giudice, M.; Capristo, A.F.; Mirra, G.; Maiello, N.; Coppola, T. Controlled double-blind study on the efficacy of clofedanol-sobrerol in the treatment of pediatric pertussis. Minerva Pediatr. 1984, 36, 1199–1206.
- Azzollini, E.; Bosi, M.; Mantegazza, M.; Piceci, E.; Careddu, P. Sobrerol (Sobrepim) administered dropwise to children with acute hypersecretory bronchopulmonary disease—A controlled trial v bromhexine. Clin. Trials J. 1990, 27, 241–249.
- Zanasi, A.; Cazzato, S.; Aprile, A.; Mazzolini, M.; Zenezini, C.; Pandolfi, P. Are antibiotics effective in treating children with acute moist cough? a retrospective study vs symptomatic therapy. Multidiscip. Respir. Med. 2012, 7, 1–5.
- Bellussi, L.; Bernocchi, D.; Ciferri, G.; Manini, G.; Passali, D. Sobrerol in the treatment of secretory otitis media in childhood. J. Int. Med. Res. 1989, 17, 277–286.
- Crosca, V.; Ajello, A.; Crosca, C.; Minniti, A. Salbutamol combined with erythromycin and sobrerol in the therapy of pertussis. Arch. Sci. Med. 1982, 139, 247–250.
