Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carolina Vieira De Almeida | -- | 2977 | 2023-07-04 07:50:55 | | | |
| 2 | Lindsay Dong | Meta information modification | 2977 | 2023-07-05 02:47:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Almeida, C.V.; Antiga, E.; Lulli, M. Probiotics in Skincare and Dermatological Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/46372 (accessed on 08 February 2026).
De Almeida CV, Antiga E, Lulli M. Probiotics in Skincare and Dermatological Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/46372. Accessed February 08, 2026.
De Almeida, Carolina Vieira, Emiliano Antiga, Matteo Lulli. "Probiotics in Skincare and Dermatological Therapy" Encyclopedia, https://encyclopedia.pub/entry/46372 (accessed February 08, 2026).
De Almeida, C.V., Antiga, E., & Lulli, M. (2023, July 04). Probiotics in Skincare and Dermatological Therapy. In Encyclopedia. https://encyclopedia.pub/entry/46372
De Almeida, Carolina Vieira, et al. "Probiotics in Skincare and Dermatological Therapy." Encyclopedia. Web. 04 July, 2023.
Copy Citation
The skin microbiota is a pivotal contributor to the maintenance of skin homeostasis by protecting it from harmful pathogens and regulating the immune system. An imbalance in the skin microbiota can lead to pathological conditions such as eczema, psoriasis, and acne. This imbalance can be triggered by different elements and dynamics such as changes in pH levels, exposure to environmental toxins, and the use of certain skincare products. Some research suggests that certain probiotic strains and their metabolites (postbiotics) may provide benefits such as improving the skin barrier function, reducing inflammation, and improving the appearance of acne-prone or eczema-prone skin.
postbiotics
probiotics
skin
skincare
1. Introduction
The last decade has seen an explosion in microbiota research, which has enabled a better understanding of its structure and function, leading to potential opportunities to develop next-generation microbiome-based drugs and diagnostic biomarkers. These studies have demonstrated that trillions of microbes live within our bodies in a deeply symbiotic relationship. They have provided evidence that microbial populations vary across body sites, driven by differences in the environment, immunological factors, and interactions between microbial species [1]. To better understand the function of the microbiome, it is fundamental to consider how microbes that live on body superficies can influence systemic performances.
The skin is the primary physical barrier that protects our bodies from potential invasion by pathogens or toxic substances, but skin does not act alone: microorganisms that live in symbiosis with us and occupy a broad array of skin niches help to protect our body against harmful situations. Microorganisms from the skin can act directly to defend the host against pathogens, control inflammation, and modulate the adaptive immune pathways [2][3][4].
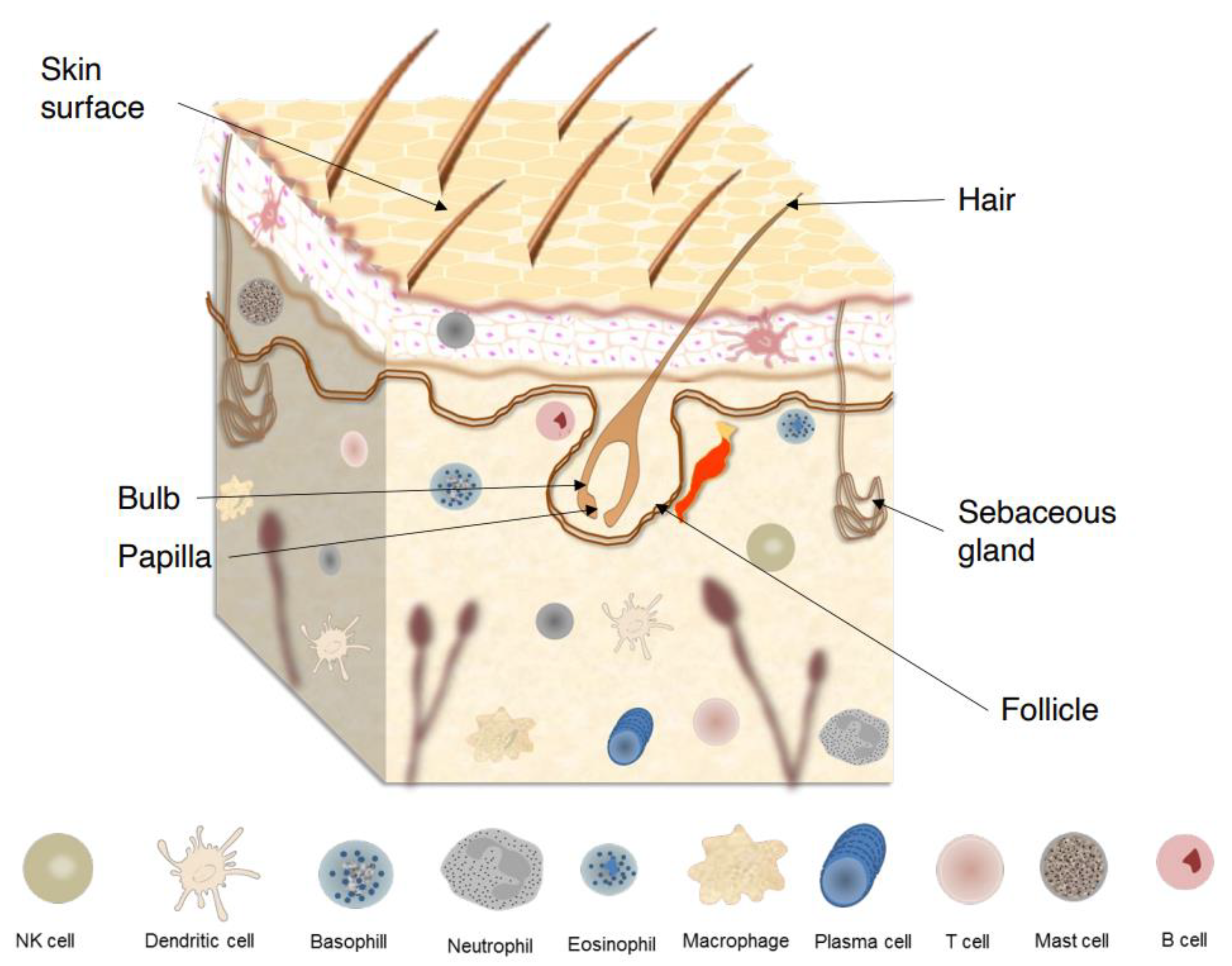
2. The Skin
The human skin comprises a variety of unique and uneven regions, with lines, ridges, and invaginations from skin appendages unevenly distributed over its surface (Figure 1). The outermost layer of epidermis is the stratum corneum (SC), which consists of piles of dead keratinocytes (corneocytes) and intercellular lipids. Due to a specific and unique type of functional cell death named corneoptosis, keratinocytes are converted into corneocytes, which remain functional in the SC and act as a functional barriers. This barriers protect the body against mechanical stresses, dehydration, toxic substances, and pathogen invasion, enabling terrestrial vertebrates succeed survive in nonaquatic environments [5][6].

Figure 1. Skin surface, skin appendages, and major components of skin immune system.
The skin is also known as ‘acid mantle’ [7], and consists of a lipid- and protein-laden cornified layer dotted with hair follicles and glands that secrete lipids, antimicrobial peptides (AMPs), enzymes, salts, and many other compounds. Over the SC, the pH is gradual, but in general, the natural skin surface, when in good conditions, presents an average pH below 5.0. This acidity is essential to maintain the balance of skin microbiota, as well as to support important physiological processes, such as the formation of the lipid barrier and SC homeostasis. In addition, the skin surface is a high salt, desiccated, and aerobic environment [2][8][9]. These odd characteristics of the skin surface make the skin physically and chemically distinct from another microbe-rich barrier sites such as the small and large intestines which are characterized by a polysaccharide-rich [10] and neutral pH surface [11].
Because it is always exposed to possible pathogens and is subject to sterile inflammation, comprising tumor immunity, allergy, and autoimmune responses, an appropriate functioning of skin defense based on complex action of a variety of complementary systems is fundamental. Complementary to the physical barrier, there is an active synthesis of gene-encoded host defense molecules such as proteases, lysozymes, antimicrobial peptides (AMPs), cytokines, and chemokines that serve as activators of the cellular and adaptive immune responses [2].
Maintenance of skin homeostasis upon inflammatory challenges requires various types of immune cells that are resident in skin or are recruited (Figure 1). For instance, in the epidermis, keratinocytes produce AMPs that exhibit direct bacteriostatic or bactericidal activity, promoting the recruitment of immune cells such as Langerhans cells (LCs) [4]. These cells are a subset of tissue-resident macrophages that reside between keratinocytes and, upon further differentiation, acquire dendritic cell (DC)-like phenotype and functions [12]. On the other hand, in the dermis, several types of innate immune cells, including dermal DCs, macrophages, mast cells, γδ T cells, and innate lymphoid cells (ILCs) are found [13].
3. The Skin Microbiota
The skin ecosystems are composed of diverse microorganisms that interact with the human body, including host epithelial and immune cells, as well as with other microorganisms sharing the same niche [14]. To establish the microbiota, the skin provides essential nutrients, such as amino acids from the hydrolysis of proteins, fatty acids from the stratum corneum, sweat, lipid hydrolysis or sebum, and lactic acids from sweat [15]. Since this symbiotic relationship between the host and commensals is crucial for several physiological processes, commensal-specific T cells distinguish resident microorganisms from pathogens to promote commensal tolerance [16][17].
Microbiota colonization begins at birth and its composition is influenced by several intrinsic (skin site, age, ethnicity, gender, intra- and interpersonal variability) and extrinsic (lifestyle, cosmetic use, use of antibiotics, hygiene routine, climate, seasonality, and geographical location) factors [18]. During puberty, it is possible to observe a decrease in the abundance of Firmicutes, including Staphylococcus and Streptococcus species, and increased predominance of Corynebacterium and Cutibacterium (previously known as Propionibacterium). On the other hand, the adult microbial composition remains constant over time despite its continuous exposure to the environment [19][20].
The skin microbiota composition is distinctive to each person as well as to each part of the body; however, Cutibacterium, Corynebacterium, and Staphylococcus represent the three most dominant microorganism genera in the human skin at the genus level [21].
The bacterial diversity is heterogeneous across skin regions due to the variety of glands and density of hair follicles, which create complex and distinct physical and chemical niches for microbial growth. Sebaceous sites, for example, have the lipophilic C. acnes and other Cutibacterium as the dominant species. Over moist sites, instead, Staphylococcus and Corynebacterium are primarily the first colonizing genera, while Lactobacillus is predominant across the female genital tract [22][23][24].
Facial skin microbiota is particularly studied due to its role in aging, acne, and rosacea. It is composed mainly by Proteobacteria (32.91%), Firmicutes (28.69%), Actinobacteria (33.07%), and Bacteroidetes (3.08%), with significant age-dependent profile variations. Subjects aged 36–52 years old have the highest Shannon index, which gives an estimate of the species diversity within a community, as recently demonstrated [25].
4. Skin Dysbiosis and Cutaneous Alterations
How homeostasis is maintained and shaped by the skin microbiota is still not clear; however, it is well established that the balance between members of skin microbial communities plays a pivotal role in guarding against cutaneous disorders. Changes in composition and the lack of balance among microbial communities is referred to as dysbiosis, a condition that may lead to the onset or progression of diseases. Pronounced dysbiosis on skin may result in several cutaneous diseases including atopic dermatitis (AD), seborrheic dermatitis (SD), rosacea, alopecia areata (AA), and acne [22][26][27].
AD affects 15–20% of children and 2–10% of adults, and results from a complex interaction between genetic susceptibility, barrier dysfunction, innate and adaptive immunity, and microbiota [28][29]. When compared to healthy controls, AD patients present a loss of diversity with increased abundance of S. aureus and depletion of S. epidermidis and Corynebacterium spp. [30][31]. Disease severity is also associated with decreased gut microbiome diversity [32][33].
A dysbiotic scenario with significant alterations of bacteria populations on the scalp skin of SD patients, another common dermatological disorder, was also demonstrated. SD symptoms include skin erythema, flaking, and pruritus. Patients with SD show reduction of Corynebacterium spp. and domination at taxa level by Firmicutes, while at genus level by Pseudomonas spp., Staphylococcus spp., and Micrococcus spp. [34].
5. Skincare and Skin Microbiota
Skin aging is a complex biological process influenced by the combination of endogenous (intrinsic) and exogenous (extrinsic) factors. The main intrinsic factors are genetics, cellular metabolism, hormones, and metabolic processes, while the main extrinsic factors are chronic light exposure, pollution, ionizing radiation, chemicals, and toxins [35].
Skin suffers progressive morphologic and physiologic decrement with increasing age and provides the first obvious evidence of the aging process. A daily skincare routine is essential to enhance smoothness, regenerate it, give strength and elasticity, as well as to prevent the degradation of collagen and elastin, which reduce the formation of wrinkles [36][37]. Moreover, various studies have examined whether cosmetics could affect skin microbiota composition and balance and have demonstrated that certain bacteria can grow by metabolizing some cosmetic ingredients [38][39]. It has been demonstrated that even with regular showering, many molecules from personal skincare and hygiene products can last on the skin for weeks after their first use. In this way, a single application of these products may alter the skin chemistry and microbiota for long periods [38].
After analyzing the effects of daily use of skincare products, a recent study has suggested that these products might improve skin biophysical parameters such as smoothness and hydration level, while pH and sebum content were maintained. The authors also suggested that a daily skincare routine might improve the microbial health of facial skin and increase the Shannon diversity [40]. As is known, high alpha diversity is considered a hallmark of healthy skin microbiota and a balanced skin microbiota is known to play an important role in skin health, as any alterations lead to the overgrowth of pathogenic strains linked to various skin diseases [41].
Another study revealed that skin metabolome and microbiome can be altered with changes in the hygiene routine, but that this alteration has responses that are specific to the individual [38]. However, as previously cited, the better conditions of skin biophysical parameters, such as barrier function, scaling, and moisturization, are found when its pH average is below 5. Use of products with high pH alkalizes the surface of the skin, which can cause irritability and an increase in dehydration, as well as changes in the microbiota composition by promoting their dispersal from the skin [9][42]. Therefore, the pH factor should be given due consideration by consumers and cosmetics producers, especially when it comes to sensitive and acne-prone skin.
6. Topical Use of Probiotics
The skin microbiota, its interactions with the environment, and the possibility of manipulating it to address cutaneous conditions have opened exciting new paths for dermatological therapies. Therefore, the cosmetic and pharmaceutical industries have been engaged in ordering solutions coming from nature, especially probiotics and postbiotics.
The use of probiotics as a potential alternative to antibiotics was previously demonstrated in the treatment of inflammatory bowel disease (IBD) [43] and several atopic conditions [44][45]. Topical probiotics were first proposed as a treatment for cutaneous diseases in 1912 to treat acne and seborrhea [46] that involves the transfer of laboratory cultured live bacteria in a dose suitable for skin, to equilibrate the skin microbiota, reestablishing the immune homeostasis [47]. Such products rely on the fact that skin immune setting is highly dynamic and can be rapidly remodeled by encounters with specific commensals. In this way, through essential interactions, topical probiotics implement both the establishment and restoration of cutaneous homeostasis [48].
It is known that, under specific conditions, probiotics can persist and successfully colonize the skin [49], inducing keratinocytes and sebocytes to produce AMPs or other metabolites that can directly inhibit or kill pathogenic microorganisms, shaping microbial communities [50][51][52], and establishing a synergistic effect which improve the ecology of skin microbial communities [53]. Considering that the first step in bacterial colonization that leads to infection is cell adhesion, the ability of probiotics to invade and adhere to keratinocytes, promoting inhibition of pathogen binding to these cells, reinforces that probiotics could be used to reduce adhesion of some pathogens to skin [54]. Moreover, the potential antimicrobial use of AMPs produced by probiotics has been shown. Indeed, it has been observed that some coagulase-negative Staphylococcus (CoNS) species, including S. hominis and S. epidermidis, can produce commensal-derived AMPs (phenol-soluble modulins and Sh-lantibiotics), which exert selective antimicrobial activity and cooperate synergistically with host-derived AMPs, such as cathelicidin LL-37, to inhibit survival of skin pathogens [50][55][56][57]. It has been proposed that by directly producing AMPs, the microbiome provides the first line of defense against microbial pathogens, synergizing with the host innate immune response as a second potent line of defense [50]. This normal microbial defense strategy against colonization and transmission of bacterial pathogens should be exploited for anti-infective therapeutics [55][56][57].
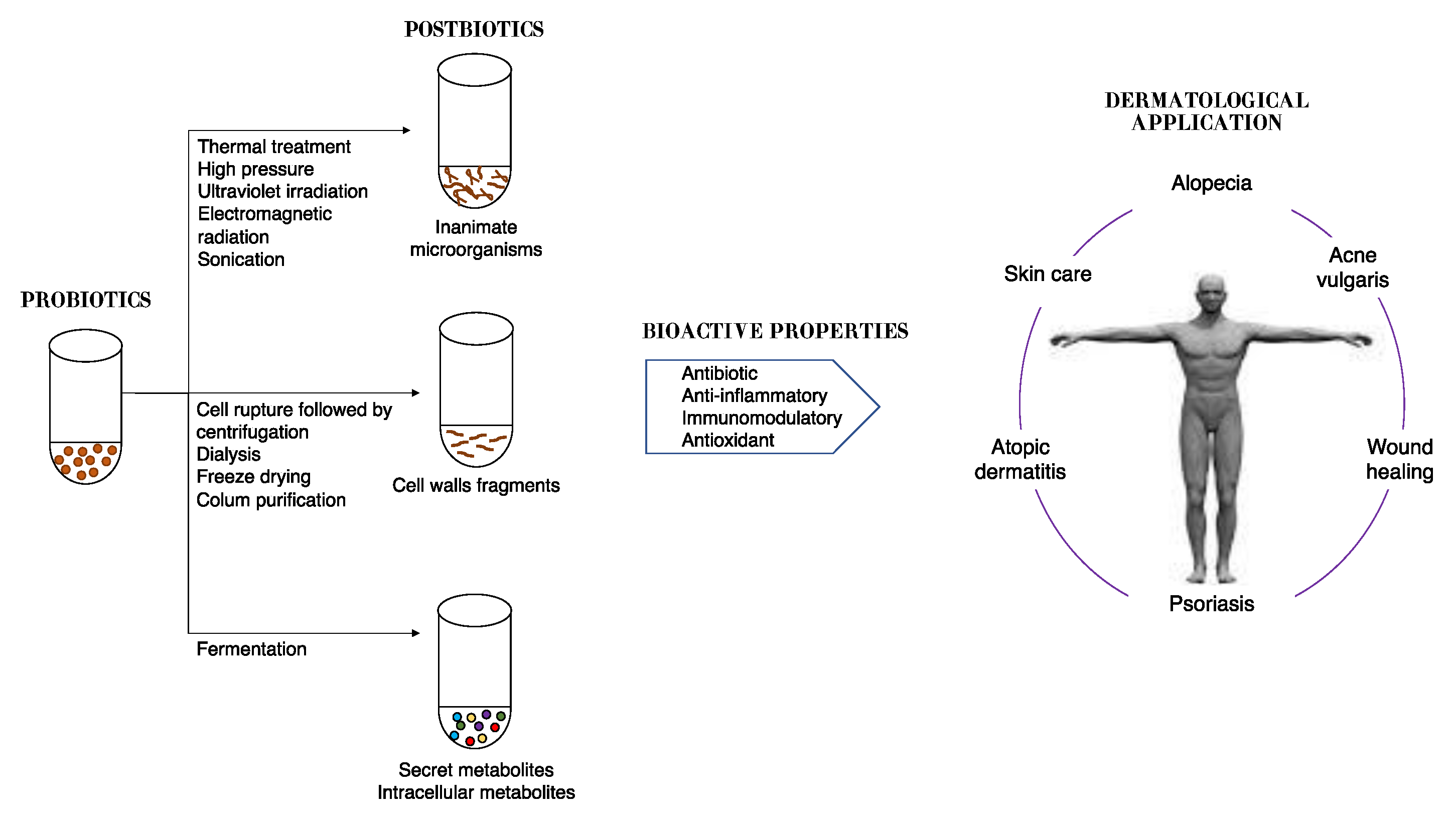
7. Topical Use Postbiotics
Dead cells present on probiotics are able to produce biological responses as effectively as their live equivalents. In fact, it was demonstrated that in adequate amounts, when administered orally or topically, dead microorganisms confer a benefit on the human or animal consume [58][59]. However, since they cannot be classified as probiotics, for dead microorganism products new terms have been coined such as postbiotics, paraprobiotics, non-viable probiotics, inactivated probiotics, tyndallized probiotics, or ghost probiotics [60][61][62]. The inactivation of viable cells could be performed by using various mechanical methods, such as sonication, thermal treatment, electromagnetic radiation, high pressure, or ultraviolet irradiation [63].
The term postbiotic was recently defined by the International Scientific Association of Probiotics and Prebiotics as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [64]; thus, they cannot colonize the host. Other components that are not required in a postbiotic, such as physiologically active microbial cellular components (cell wall fragments or enzymes) or functional bioactive substances (products or metabolic byproducts) such as vitamins, short-chain fatty acids (SCFAs), proteins and antibiotics, can contribute substantively to the complexity and functionality of the postbiotic preparation [65]. It is important to highlight that purified metabolites and purified cell components have specific chemical names of their own that can be used; thus, they do not fall under the scope of postbiotics, and should be referred to as microbe-derived substances.
Therefore, postbiotics can be defined as cell-free supernatants without metabolite specification/individualization, cell wall components and/or intracellular compounds (Figure 2). These compounds have many potential health benefits including anti-inflammatory, antioxidant, immunomodulatory, antimicrobial, and anti-ageing/anti-senescence activities [66].

Figure 2. Schematic representation of approaches in postbiotic production and their benefits to skin health.
Though evidence of microbiota modulation by postbiotics in humans is limited, due to the benefits overcited, the term postbiotic is emerging on commercial products for humans and animals [59]. Moreover, great effects on the skin have been seen, such as improved skin moisturizing [67], prevention of wrinkle formation [68], and amelioration of atopic dermatitis [69][70].
The topical application of postbiotics from L. fermentum, L. reuteri, and Bacillus subtilis natto, when associated with a cold cream, for example, is being considered as a novel therapeutic approach to accelerate the wound healing process, with an earlier complete epithelization and absence of skin inflammation in the group treated with L. reuteri [71]. In vitro antioxidant assays showed significant free radical scavenging activity of a cell-free, casein-free supernatant of L. helveticus strain fermented milk against UVB-induced skin photodamage. The melanin production by melanocytes cells in vitro and the expression of proteins needed for melanin synthesis were inhibited by supernatant.
The topical use of S. thermophiles lysate containing sphingomyelinase, an enzyme that converts sphingomyelin to phosphocholine and ceramide, demonstrated a significant increase in stratum corneum ceramide levels, improved the lipid barrier and reduced water loss [72]. A postbiotic from L. plantarum K8 strain lysate was recently suggested as a functional ingredient for moisturizing products, since it was able to increase the mRNA expression levels of moisturizing factors, including HAS2 and AQP3 [73].
The LactoSporin® formulation, a cream containing cell-free supernatants of Bacillus coagulans and inactivated cells of Bacillus longum, demonstrated effectiveness in mild-to-moderate acne lesions and other seborrheic conditions in both male and female subjects, due to its ability to reduce sebaceous secretion and oily, greasy nature of the skin even better than benzoyl peroxide [74].
8. Use of Oral Probiotics and Postbiotics for Ameliorating Skin Health
There is a relationship between the gut microbiome and skin health, termed the gut–skin axis. The gut–skin axis results from the resemblance between these organs: both are highly innervated and vascularized, and they are essential for immune and neuroendocrine function [75]. There are some interesting similarities between inner surface of the gut and the surface of the skin: both are covered by epithelial cells, which maintain an important link between the internal body and the external environment, acting as a first line of defense and thus preventing the entry of microorganisms [76].
Inflammatory skin diseases can be associated with disruptions of gut microbiome mediated by metabolites released by the microorganisms, as well as increased inflammatory mediators [77][78]. There is growing evidence supporting that intestinal dysbiosis is observed in common inflammatory skin diseases such as atopic dermatitis, psoriasis, rosacea, and acne vulgaris [78], which increases the potential of oral probiotics as a treatment option for skin disorders.
Oral probiotics are a group of living microorganisms that could change the gut microbiota and induce a protective effect on specific skin cells by inducing a series of immune and inflammatory responses. In recent years, several studies have indicated that the use of oral probiotics is beneficial to the skin. For example, the use of a probiotic consisting of Lactobacillus acidophilus, Lactobacillus delbrueckii subspecies bulgaricus, and Bifidobacterium bifidum was shown to be similarly effective to minocycline 100 mg daily for acne [79].
Skin photoaging is mainly caused by UVR exposition; however, it is also closely associated with changes in metabolic capacity, cofactor and vitamin metabolism, antibiotic biosynthesis, glycolipid metabolism, and fatty acid biosynthesis. A growing amount of evidence associates oral probiotics with skin photoaging control by positively modulating gut–skin microbial interaction, reducing the oxidative stress level, inhibiting the inflammatory cascade, maintaining immune homeostasis, and inhibiting extracellular matrix remodeling [80].
Oral consumption of some postbiotics has been shown to respect microbiota equilibrium and to restore/improve the skin barrier integrity through antioxidant activity and inhibition of enzymes associated with extracellular matrix disintegration. Thus, it is postulated that postbiotics can improve UV protection, delaying the ageing process of skin cells [81].
Efficacy limitations of topical strategies to treat skin alterations have emerged, thus prompting the evaluation of therapeutic oral probiotics administration as an efficient alternative. Modulating the gut microbiota with symbiotics (probiotics plus prebiotics) or prebiotics also demonstrated great effects on skin health. Oral administration of Lactocare®, a symbiotic formulation, when associated with topical administration of hydrocortisone demonstrated improvement in the psoriasis indexes [82].
References
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front. Microbiol. 2022, 13, 825338.
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455.
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382.
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119.
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340.
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280.
- Schade, H.; Marchionini, A. Der Säuremantel der Haut nach Gaskettenmessngen. Klin Wochenschr. 1928, 7, 12–14.
- Rieger, M.M. The pH of the SC: An update. Cosm. Toil. 2000, 115, 43–45.
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370.
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32.
- Firrman, J.; Liu, L.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. EMS Microbiol. Ecol. 2022, 98, fiac038.
- Hoeffel, G.; Wang, Y.; Greter, M.; See, P.; Teo, P.; Malleret, B.; Leboeuf, M.; Low, D.; Oller, G.; Almeida, F.; et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012, 209, 1167–1181.
- Tong, P.L.; Roediger, B.; Kolesnikoff, N.; Biro, M.; Tay, S.S.; Jain, R.; Shaw, L.E.; Grimbaldeston, M.A.; Weninger, W. The skin immune atlas: Three-dimensional analysis of cutaneous leukocyte subsets by multiphoton microscopy. J. Investig. Dermatol. 2015, 135, 84–93.
- Flowers, L.; Grice, E.A. The skin microbiota: Balancing risk and reward. Cell Host Microbe 2020, 28, 190–200.
- Wilson, M. The Indigenous Microbiota of the Skin. The Human Microbiota in Health and Disease: An Ecological and Community-Based Approach; Garland Science Taylor & Francis: Cambridge, UK, 2018; pp. 86–95.
- Swaney, M.H.; Kalan, L.R. Living in your skin: Microbes, molecules, and mechanisms. Infect. Immun. 2021, 89, e00695-20.
- Harrison, O.J.; Linehan, J.L.; Shih, H.Y.; Bouladoux, N.; Han, S.J.; Smelkinson, M.; Sen, S.K.; Byrd, A.L.; Enamorado, M.; Yao, C.; et al. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 2019, 363, eaat6280.
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Saez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell Infect. Microbiol. 2022, 12, 834135.
- Oh, J.; Conlan, S.; Polley, E.C.; Segre, J.A.; Kong, H.H. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012, 4, 77.
- Oh, J.; Byrd, A.L.; Park, M.; NISC Comparative Sequencing Program; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866.
- Scharschmidt, T.C.; Fischbach, M.A. What lives on our skin: Ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. emporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859.
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192.
- Russo, E.; Di Gloria, L.; Cerboneschi, M.; Smeazzetto, S.; Baruzzi, G.P.; Romano, F.; Ramazzotti, M.; Amedei, A. Facial Skin Microbiome: Aging-Related Changes and Exploratory Functional Associations with Host Genetic Factors, a Pilot Study. Biomedicines 2023, 11, 684.
- Sanford, J.A.; Gallo, R.L. Functions of the skin microbiota in health and disease. Semin. Immunol. 2013, 25, 370–377.
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in healthy skin, update for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047.
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651.
- Lunjani, N.; Hlela, C.; O’Mahony, L. Microbiome and skin biology. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 328–333.
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019, 10, 4703.
- Zollner, T.M.; Wichelhaus, T.A.; Hartung, A.; Von Mallinckrodt, C.; Wagner, T.O.; Brade, V.; Kaufmann, R. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clin. Exp. Allergy. 2000, 30, 994–1000.
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353.
- Hu, C.; van Meel, E.R.; Medina-Gomez, C.; Kraaij, R.; Barroso, M.; Kiefte-de Jong, J.; Radjabzadeh, D.; Pasmans, S.G.M.A.; de Jong, N.W.; de Jongste, J.C.; et al. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J. Allergy Clin. Immunol. 2021, 148, 612–620.
- Dityen, K.; Soonthornchai, W.; Kueanjinda, P.; Kullapanich, C.; Tunsakul, N.; Somboonna, N.; Wongpiyabovorn, J. Analysis of cutaneous bacterial microbiota of Thai patients with seborrheic dermatitis. Exp. Dermato. 2022, 31, 1949–1955.
- Cevenini, E.; Invidia, L.; Lescai, F.; Salvioli, S.; Tieri, P.; Castellani, G.; Franceschi, C. Human models of aging and longevity. Expert. Opin. Biol. Ther. 2008, 8, 1393–1405.
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol. Dermatoendocrinol. 2012, 4, 308–319.
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372.
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47.
- Lee, H.J.; Jeong, S.E.; Lee, S.; Kim, S.; Han, H.; Jeon, C.O. Effects of cosmetics on the skin microbiome of facial cheeks with different hydration levels. Microbiologyopen 2018, 7, e00557.
- Hwang, B.K.; Lee, S.; Myoung, J.; Hwang, S.J.; Lim, J.M.; Jeong, E.T.; Park, S.G.; Youn, S.H. Effect of the skincare product on facial skin microbial structure and biophysical parameters: A pilot study. Microbiologyopen 2021, 10, e1236.
- Ciardiello, T.; Pinto, D.; Marotta, L.; Giuliani, G.; Rinaldi, F. Effects of Fermented Oils on Alpha-Biodiversity and Relative Abundance of Cheek Resident Skin Microbiota. Cosmetics 2020, 7, 34.
- Tarun, J.; Susan, J.; Suria, J.; Susan, V.J.; Criton, S. Evaluation of pH of Bathing Soaps and Shampoos for Skin and Hair Care. Indian J. Dermatol. 2014, 59, 442–444.
- Gionchetti, P.; Rizzello, F.; Lammers, K.; Morselli, C.; Sollazzi, L.; Davies, S.; Tambasco, R.; Calabrese, C.; Campieri, M. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J. Gastroenterol. 2006, 12, 3306–3313.
- Gill, H.S. Probiotics and human health: A clinical perspective. Postgrad. Med. J. 2004, 80, 516–526.
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079.
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brainskin axis—Back to the future? Gut Pathog. 2011, 3, 1.
- Romagnani, R. Coming back to a missing immune deviation as the main explanatory mechanism for the hygiene hypothesis. J. Allergy Clin. Immunol. 2007, 119, 1511–1513.
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediat. Inflamm. 2017, 2017, 5095293.
- Lizardo, M.P.; Tavaria, F.K. Probiotic growth in skin-like conditions. AIMS Microbiol. 2022, 8, 388–402.
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; Latif, H.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680.
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 2018, 4, eaao4502.
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108.
- Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin microbiome modulation induced by probiotic solutions. Microbiome 2019, 7, 95.
- Lizardo, M.; Magalhães, R.M.; Tavaria, F.K. Probiotic Adhesion to Skin Keratinocytes and Underlying Mechanisms. Biology 2022, 11, 1372.
- Nakatsuji, T.; Gallo, R.L. The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 263–269.
- Cogen, A.L.; Yamasaki, K.; Sanchez, K.M.; Dorschner, R.A.; Lai, Y.; MacLeod, D.T.; Torpey, J.W.; Otto, M.; Nizet, V.; Kim, J.E.; et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Investig. Dermatol. 2010, 130, 192–200.
- Cogen, A.L.; Yamasaki, K.; Muto, J.; Sanchez, K.M.; Crotty Alexander, L.; Tanios, J.; Lai, Y.; Kim, J.E.; Nizet, V.; Gallo, R.L. Taphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS ONE 2010, 5, e8557.
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168.
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274.
- Chuang, L.; Wu, K.G.; Pai, C.; Hsieh, P.S.; Tsai, J.J.; Yen, J.H.; Lin, M.Y. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J. Agric. Food Chem. 2007, 55, 11080–11086.
- Jorjão, A.L.; de Oliveira, F.E.; Leão, M.V.; Carvalho, C.A.; Jorge, A.O.; de Oliveira, L.D. Live and Heat-Killed Lactobacillus rhamnosus ATCC 7469 May Induce Modulatory Cytokines Profiles on Macrophages RAW 264.7. Sci. World J. 2015, 2015, 716749.
- Wilcox, H.; Carr, C.; Seney, S.; Reid, G.; Burton, J.P. Expired probiotics: What is really in your cabinet? FEMS Microbes. 2020, 1, xtaa007.
- Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114.
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667.
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673.
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current postbiotics in the cosmetic market-an update and development opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891.
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872.
- Im, A.R.; Lee, B.; Kang, D.J.; Chae, S. Skin. moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food 2018, 21, 1016–1023.
- Choi, E.J.; Iwasa, M.; Han, K.I.; Kim, W.J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.S.; Kim, E.K. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients 2016, 8, 146.
- Lee, S.H.; Yoon, J.M.; Kim, Y.H.; Jeong, D.G.; Park, S.; Kang, D.J. Therapeutic effect of tyndallized Lactobacillus rhamnosus IDCC 3201 on atopic dermatitis mediated by down-regulation of immunoglobulin E in NC/Nga mice. Microbiol. Immunol. 2016, 60, 468–476.
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A Novel Effective Formulation of Bioactive Compounds for Wound Healing: Preparation, In Vivo Characterization, and Comparison of Various Postbiotics Cold Creams in a Rat Model. Evid. Based Complement. Alternat Med. 2021, 2021, 8577116.
- Di Marzio, L.; Cinque, B.; Cupelli, F.; De Simone, C.; Cifone, M.G.; Giuliani, M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008, 21, 137–143.
- Kim, H.; Jeon, B.; Kim, W.J.; Chung, D.K. Effect of paraprobiotic prepared from Kimchi-derived Lactobacillus plantarum K8 on skin moisturizing activity in human keratinocyte. J. Funct. Foods 2020, 75, 104244.
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel Topical Application of a Postbiotic, Lactosporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate Its Efficacy, Tolerabili and Safety. Cosmetics 2020, 7, 70.
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176.
- Shaykhiev, R.; Bals, R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J. Leukoc. Biol. 2007, 82, 1–15.
- Le, S.T.; Toussi, A.; Maverakis, N.; Marusina, A.I.; Barton, V.R.; Merleev, A.A.; Luxardi, G.; Raychaudhuri, S.P.; Maverakis, E. The cutaneous and intestinal microbiome in psoriatic disease. Clin. Immunol. 2020, 218, 108537.
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218.
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J. Cutan. Med. Surg. 2013, 17, 114–122.
- Teng, Y.; Huang, Y.; Danfeng, X.; Tao, X.; Fan, Y. The Role of Probiotics in Skin Photoaging and Related Mechanisms: A Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2455–2464.
- Majeed, M.; Nagabhushanam, K.; Paulose, S.; Rajalakshmi, H.R.; Mundkur, L. A Randomized Double-Blind, Placebo-Controlled Study to Evaluate the Anti-Skin-Aging Effect of LactoSporin—The Extracellular Metabolite from Bacillus coagulans (Weizmannia coagulans) MTCC 5856 in Healthy Female Volunteers. Clin. Cosmet. Investig. Dermatol. 2023, 16, 769–782.
- Akbarzadeh, A.; Alirezaei, P.; Doosti-Irani, A.; Mehrpooya, M.; Nouri, F. The Efficacy of Lactocare® Synbiotic on the Clinical Symptoms in Patients with Psoriasis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Dermatol. Res. Pract. 2022, 2022, 4549134.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
867
Revisions:
2 times
(View History)
Update Date:
05 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




