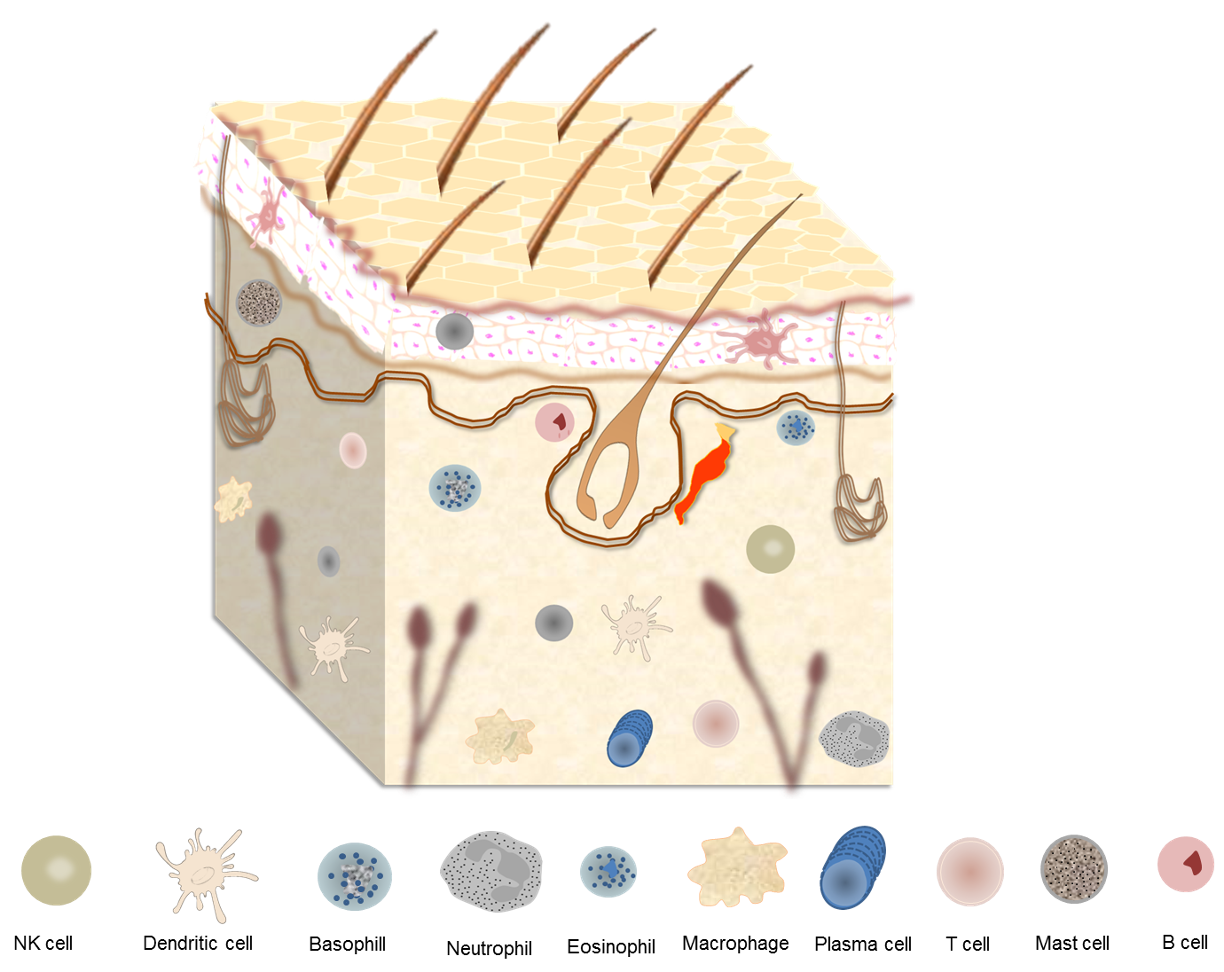
Another study revealed that skin metabolome and microbiome can be altered with changes in the hygiene routine, but that this alteration has responses that are specific to the individual [
]. However, as previously cited, the better conditions of skin biophysical parameters, such as barrier function, scaling, and moisturization, are found when its pH average is below 5. Use of products with high pH alkalizes the surface of the skin, which can cause irritability and an increase in dehydration, as well as changes in the microbiota composition by promoting their dispersal from the skin [
]. Therefore, the pH factor should be given due consideration by consumers and cosmetics producers, especially when it comes to sensitive and acne-prone skin.
The skin microbiota, its interactions with the environment, and the possibility of manipulating it to address cutaneous conditions have opened exciting new paths for dermatological therapies. Therefore, the cosmetic and pharmaceutical industries have been engaged in ordering solutions coming from nature, especially probiotics and postbiotics.
The use of probiotics as a potential alternative to antibiotics was previously demonstrated in the treatment of inflammatory bowel disease (IBD) [
77] and several atopic conditions [
78,
79]. Topical probiotics were first proposed as a treatment for cutaneous diseases in 1912 to treat acne and seborrhea [
80] that involves the transfer of laboratory cultured live bacteria in a dose suitable for skin, to equilibrate the skin microbiota, reestablishing the immune homeostasis [
81]. Such products rely on the fact that skin immune setting is highly dynamic and can be rapidly remodeled by encounters with specific commensals. In this way, through essential interactions, topical probiotics implement both the establishment and restoration of cutaneous homeostasis [
82].
It is known that, under specific conditions, probiotics can persist and successfully colonize the skin [
83], inducing keratinocytes and sebocytes to produce AMPs or other metabolites that can directly inhibit or kill pathogenic microorganisms, shaping microbial communities [
84,
85,
86], and establishing a synergistic effect which improve the ecology of skin microbial communities [
87]. Considering that the first step in bacterial colonization that leads to infection is cell adhesion, the ability of probiotics to invade and adhere to keratinocytes, promoting inhibition of pathogen binding to these cells, reinforces that probiotics could be used to reduce adhesion of some pathogens to skin [
88]. Moreover, the potential antimicrobial use of AMPs produced by probiotics has been shown. Indeed, it has been observed that some coagulase-negative
Staphylococcus (CoNS) species, including
S. hominis and
S. epidermidis, can produce commensal-derived AMPs (phenol-soluble modulins and
Sh-lantibiotics), which exert selective antimicrobial activity and cooperate synergistically with host-derived AMPs, such as cathelicidin LL-37, to inhibit survival of skin pathogens [
84,
89,
90,
91]. It has been proposed that by directly producing AMPs, the microbiome provides the first line of defense against microbial pathogens, synergizing with the host innate immune response as a second potent line of defense [
84]. This normal microbial defense strategy against colonization and transmission of bacterial pathogens should be exploited for anti-infective therapeutics [
89,
90,
91].
7. Topical Use Postbiotics
Dead cells present on probiotics are able to produce biological responses as effectively as their live equivalents. In fact, it was demonstrated that in adequate amounts, when administered orally or topically, dead microorganisms confer a benefit on the human or animal consume [
104,
105]. However, since they cannot be classified as probiotics, for dead microorganism products new terms have been coined such as postbiotics, paraprobiotics, non-viable probiotics, inactivated probiotics, tyndallized probiotics, or ghost probiotics [
106,
107,
108]. The inactivation of viable cells could be performed by using various mechanical methods, such as sonication, thermal treatment, electromagnetic radiation, high pressure, or ultraviolet irradiation [
109].
The term postbiotic was recently defined by the International Scientific Association of Probiotics and Prebiotics as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [
110]; thus, they cannot colonize the host. Other components that are not required in a postbiotic, such as physiologically active microbial cellular components (cell wall fragments or enzymes) or functional bioactive substances (products or metabolic byproducts) such as vitamins, short-chain fatty acids (SCFAs), proteins and antibiotics, can contribute substantively to the complexity and functionality of the postbiotic preparation [
111]. It is important to highlight that purified metabolites and purified cell components have specific chemical names of their own that can be used; thus, they do not fall under the scope of postbiotics, and should be referred to as microbe-derived substances.
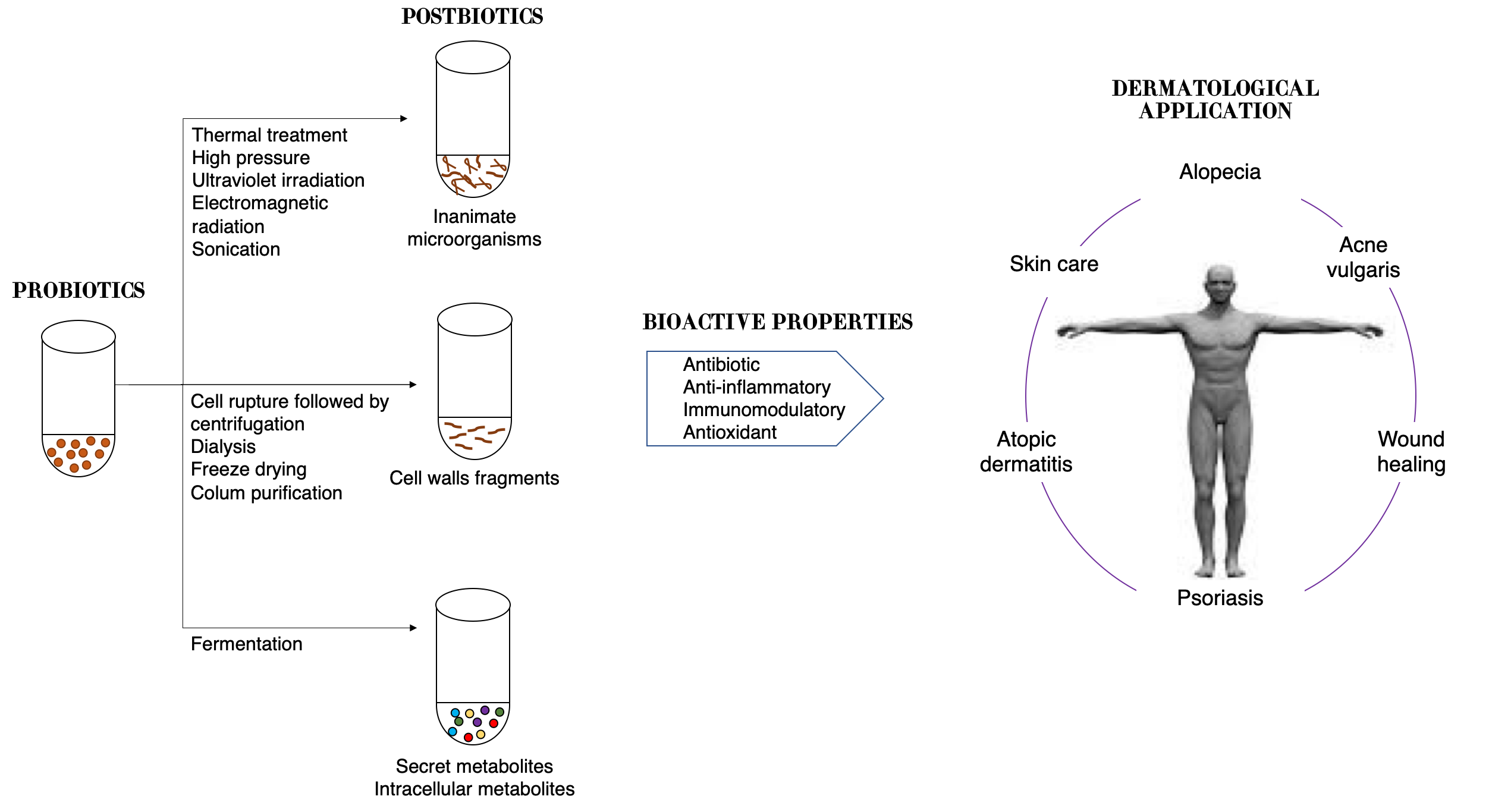
Therefore, postbiotics can be defined as cell-free supernatants without metabolite specification/individualization, cell wall components and/or intracellular compounds (
Figure 2). These compounds have many potential health benefits including anti-inflammatory, antioxidant, immunomodulatory, antimicrobial, and anti-ageing/anti-senescence activities [
112].
Figure 2. Schematic representation of approaches in postbiotic production and their benefits to skin health.
Though evidence of microbiota modulation by postbiotics in humans is limited, due to the benefits overcited, the term postbiotic is emerging on commercial products for humans and animals [
105]. Moreover, great effects on the skin have been seen, such as improved skin moisturizing [
115], prevention of wrinkle formation [
116], and amelioration of atopic dermatitis [
117,
118].
The topical application of postbiotics from
L. fermentum,
L. reuteri, and
Bacillus subtilis natto, when associated with a cold cream, for example, is being considered as a novel therapeutic approach to accelerate the wound healing process, with an earlier complete epithelization and absence of skin inflammation in the group treated with
L. reuteri [
119]. In vitro antioxidant assays showed significant free radical scavenging activity of a cell-free, casein-free supernatant of
L. helveticus strain fermented milk against UVB-induced skin photodamage. The melanin production by melanocytes cells in vitro and the expression of proteins needed for melanin synthesis were inhibited by supernatant.
The topical use of
S. thermophiles lysate containing sphingomyelinase, an enzyme that converts sphingomyelin to phosphocholine and ceramide, demonstrated a significant increase in stratum corneum ceramide levels, improved the lipid barrier and reduced water loss [
122]. A postbiotic from
L. plantarum K8 strain lysate was recently suggested as a functional ingredient for moisturizing products, since it was able to increase the mRNA expression levels of moisturizing factors, including HAS2 and AQP3 [
123].
The LactoSporin
® formulation, a cream containing cell-free supernatants of
Bacillus coagulans and inactivated cells of
Bacillus longum, demonstrated effectiveness in mild-to-moderate acne lesions and other seborrheic conditions in both male and female subjects, due to its ability to reduce sebaceous secretion and oily, greasy nature of the skin even better than benzoyl peroxide [
128].
8. Use of Oral Probiotics and Postbiotics for Ameliorating Skin Health
There is a relationship between the gut microbiome and skin health, termed the gut–skin axis. The gut–skin axis results from the resemblance between these organs: both are highly innervated and vascularized, and they are essential for immune and neuroendocrine function [
132]. There are some interesting similarities between inner surface of the gut and the surface of the skin: both are covered by epithelial cells, which maintain an important link between the internal body and the external environment, acting as a first line of defense and thus preventing the entry of microorganisms [
133].
Inflammatory skin diseases can be associated with disruptions of gut microbiome mediated by metabolites released by the microorganisms, as well as increased inflammatory mediators [
136,
137]. There is growing evidence supporting that intestinal dysbiosis is observed in common inflammatory skin diseases such as atopic dermatitis, psoriasis, rosacea, and acne vulgaris [
137], which increases the potential of oral probiotics as a treatment option for skin disorders.
Oral probiotics are a group of living microorganisms that could change the gut microbiota and induce a protective effect on specific skin cells by inducing a series of immune and inflammatory responses. In recent years, several studies have indicated that the use of oral probiotics is beneficial to the skin. For example, the use of a probiotic consisting of
Lactobacillus acidophilus,
Lactobacillus delbrueckii subspecies
bulgaricus, and
Bifidobacterium bifidum was shown to be similarly effective to minocycline 100 mg daily for acne [
138].
Skin photoaging is mainly caused by UVR exposition; however, it is also closely associated with changes in metabolic capacity, cofactor and vitamin metabolism, antibiotic biosynthesis, glycolipid metabolism, and fatty acid biosynthesis. A growing amount of evidence associates oral probiotics with skin photoaging control by positively modulating gut–skin microbial interaction, reducing the oxidative stress level, inhibiting the inflammatory cascade, maintaining immune homeostasis, and inhibiting extracellular matrix remodeling [
141].
Oral consumption of some postbiotics has been shown to respect microbiota equilibrium and to restore/improve the skin barrier integrity through antioxidant activity and inhibition of enzymes associated with extracellular matrix disintegration. Thus, it is postulated that postbiotics can improve UV protection, delaying the ageing process of skin cells [
142].
Efficacy limitations of topical strategies to treat skin alterations have emerged, thus prompting the evaluation of therapeutic oral probiotics administration as an efficient alternative. Modulating the gut microbiota with symbiotics (probiotics plus prebiotics) or prebiotics also demonstrated great effects on skin health. Oral administration of Lactocare
®, a symbiotic formulation, when associated with topical administration of hydrocortisone demonstrated improvement in the psoriasis indexes [
144].