Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. Classification and Biosynthesis of Flavonoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/46367 (accessed on 01 March 2026).
Chen S, Wang X, Cheng Y, Gao H, Chen X. Classification and Biosynthesis of Flavonoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/46367. Accessed March 01, 2026.
Chen, Shen, Xiaojing Wang, Yu Cheng, Hongsheng Gao, Xuehao Chen. "Classification and Biosynthesis of Flavonoids" Encyclopedia, https://encyclopedia.pub/entry/46367 (accessed March 01, 2026).
Chen, S., Wang, X., Cheng, Y., Gao, H., & Chen, X. (2023, July 04). Classification and Biosynthesis of Flavonoids. In Encyclopedia. https://encyclopedia.pub/entry/46367
Chen, Shen, et al. "Classification and Biosynthesis of Flavonoids." Encyclopedia. Web. 04 July, 2023.
Copy Citation
Flavonoids are mainly found in plant cell vacuoles in the form of C-glycosides or O-glycosides. The basic molecular structure of flavonoids depends upon their basic C6–C3–C6 skeleton. Flavonoids are classified into seven subclasses based on modifications to their basic skeletons; these subclasses include flavones, flavanones, isoflavones, flavonols, chalcones, flavanols, and anthocyanins.
flavonoids
biosynthesis pathway
classification
biological activity
application
1. Flavonoid Classification
1.1. Flavones
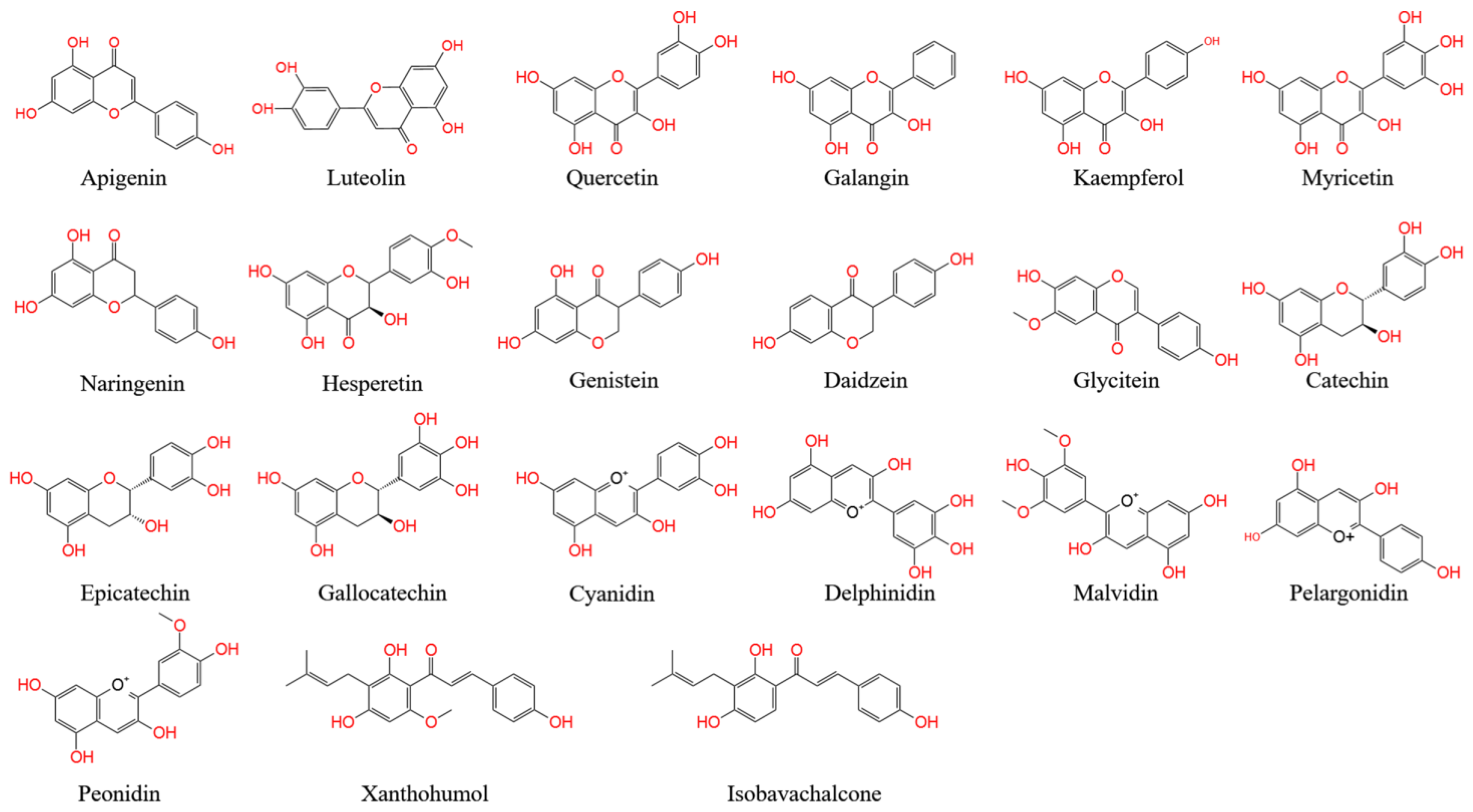
Flavones, one of the largest classes of flavonoids, consist of 4H-chromen-4-one bearing a phenyl substituent at position 2. Flavones mostly occur as 7-O-glycosides, which are found in celery, parsley, red pepper, chamomile, mint, and ginkgo [1][2][3]. Apigenin and luteolin are two common flavones (Figure A1). In nature, apigenin is usually found in a glycosylated form, with a sugar moiety attached to the tricyclic core structure via hydroxyl groups (O-glycosides) or directly to carbon (C-glycosides) [4]. The principal ingredients of apigenin are glycosylated apiin, apigenin, vitexin, isovitexin, or rhoifolin. Apigenin can scavenge free radicals and regulate antioxidant enzyme activity in pancreatic cells, and apigenin can decrease inflammation in cancer, neuroinflammation, and cardiovascular diseases [5][6].
1.2. Flavonols
Flavonols, also called 3-hydroxy flavone, can be identified by specific substitutions in their A- and B-rings, which are connected by a three-carbon chain [7]. Flavonols possess hydroxyl groups at positions 5 and 7 in the A-ring and are mainly present in epidermal cells to protect DNA against UV-induced damage [8]. Four types of flavonol compounds (quercetin, galangin, kaempferol, and myricetin) are mainly distributed in vegetables and fruits, such as asparagus, onions, lettuce, broccoli, tomato, and apples (Figure A1) [9]. Flavonols exhibit interesting biological activities, including antioxidant, antibacterial, cardioprotective, anticancer, and antiviral activities. Dietary flavonols can significantly decrease the risk of gastric cancer in smokers and in women (Figure A1).
1.3. Flavanones
Flavanones (dihydro-flavones) possess a saturated C-ring [10]. The saturated double bond between positions 2 and 3 in the C-ring represents the only structural difference between flavanones and other flavonoid compounds [11]. Flavanones are mainly distributed in citrus fruits, including oranges, lemons, mandarins, grapefruits, clementines, and limes [12]. Flavanones contain hydroxyl groups at positions 5 and 7 in the A-ring and possess hydroxyl/methoxy substituents at the C3 or C4 positions of the B-ring [13]. The defining characteristic of flavanones is a disaccharidic moiety linked to the seven positions of aglycone [14]. Depending on their structural differences, flavanones can occur in the form of naringin, naringenin, hesperidin, hesperetin, pinocembrin, likvirtin, and eriodictyol [15]. Among them, naringenin and hesperetin, as the main dietary flavanones, occur almost exclusively in citrus fruits (Figure A1) [12][16]. Naringin can increase the activity of antioxidant enzymes (CAT, PON, GPx, and SOD) and enhance the immune system. Furthermore, naringenin and hesperetin have been shown to recover impaired thyroid function in rats.
1.4. Isoflavonoids
Isoflavones have a B-ring at the C3 position of the heterocyclic C-ring of the diphenylpropane (C6–C3–C6) backbone, which represents their only chemical structural difference from other flavonoids [17]. Isoflavonoids are characteristic metabolites of leguminous plants and play essential roles in nodule induction and microbial signaling in legumes [18][19]. Isoflavones are classified into three groups: genistein, daidzein, and glycitein (Figure A1) [20]. The molecular structure of isoflavones is similar to that of animal estrogens. Isoflavones are phytoestrogens that exhibit potent estrogenic activity. Phytoestrogens are similar in structure to the human female hormone 17-β-estradiol in that they bind to estrogen receptors [21]. In addition, isoflavones possess a strong antioxidant activity, which can decrease the risk of cancers by inhibiting free radical-induced DNA damage [21].
1.5. Flavanols
Flavanols, also called catechins or flavan-3-ols, are characterized by a hydroxyl group at position 3 in the C-ring [22]. Flavanols lack a double bond between positions 2 and 3 in the C-ring [23]. Several flavanols, including catechin, gallocatechin 3-gallate, gallocatechin, epicatechin, epicatechin 3-gallate, catechin 3-gallate, and epicatechin 3-gallate, are widely distributed in many fruits (e.g., apples, bananas, pears, and blueberries) [24][25]. Flavanols can protect blood vessels against tobacco by increasing the content of NO in blood vessels. A flavanol-rich diet can facilitate the permanent improvement of endothelial function and prevent the development of cardiovascular diseases [26][27].
1.6. Anthocyanins
Anthocyanins, as glycosylated polyphenolic compounds, are a group of soluble vacuolar pigments that possess a range of colors, from orange, red, and purple to blue, depending on the pH of the micro-environment of the flowers, seeds, fruits, and vegetative tissues [28]. The position and number of hydroxyl and methoxyl groups present as substituents in the flavylium structure result in different anthocyanins (Figure A1). Thus, over 650 anthocyanins have been identified in many plants [29]; these are grouped into five items, including cyanidin, delphinidin, malvidin, pelargonidin, and peonidin, and their corresponding derivatives [30]. Anthocyanins are mainly found in the outer cell layer of various fruits and vegetables, such as blackcurrants, grapes, and berries [31][32]. The antioxidant ability of anthocyanins is associated with their ring orientation and the position and number of free hydroxyls around the pyrone ring. Anthocyanins play important roles in visual acuity, cholesterol decomposition, and the reduced risk of cardiovascular disease in humans [33][34]. In addition, anthocyanins are commonly used as food colorants.
1.7. Chalcones
Chalcones (1,3-diaryl-2-propen-1-ones) are natural open-chain flavonoids, carrying up to three modified or unmodified C5-, C10-, and C15-prenyl moieties on both their A and B-rings. These bioactive products are widely distributed in the Fabaceae, Moraceae, Zingiberaceae, and Cannabaceae families [5]. They exhibit a wide spectrum of pharmacological effects, including antioxidant, antibacterial, anthelmintic, antiulcer, antiviral, antiprotozoal, and anticancer effects [35]. Chalcones are precursors of flavonoids and isoflavonoids. Their structural features are easily constructed from simple aromatic compounds. Their prominent bioactivity has inspired the synthesis of chalcone analogs, as well as minor structural modifications to natural chalcones; these compounds form a large collection of bioactive chalcone derivatives [36]. Xanthohumol and isbavirachalone are two representative derivatives that exhibit abundant biological and pharmacological activity (Figure A1) [33].
Generally, the number and position of –OH groups have a great influence on flavonoid bioactivity. The –OH groups can link to the carbon atoms of the benzene ring (3,5,7, and 3′,4′-dihydroxy substitution pattern), which directly determines the bioactivity of flavonoids. Moreover, the position of the –OH group also influenced the flavonoid bioactivity. The most effective radical scavengers are flavonoids with the 3′,4′-dihydroxy substitution pattern on the B-ring and/or hydroxyl group at the C-3 position. In addition, the C2–C3 double bond is not necessary for high activity. Flavanols lacking the C2–C3 double bond displayed strong activity. The presence of a 3 –OH group significantly enhances the bioactivity.
2. Flavonoid Biosynthesis in Plants
2.1. Flavonoid Biosynthetic Pathways
Flavonoid synthesis occurs at the junction of the shikimate pathway and the acetate pathway. The former can generate p-coumaroyl-CoA, and the latter regulates C2-chain elongation [37] (Figure 1). Phenylalanine ammonia-lyase (PAL) deaminates phenylalanine to ammonia and cinnamic acid [38]. Then, C4H (cinnamic acid 4-hydroxylase) catalyzes the production of 4-coumaric acid [39], and 4CL (4-coumaric acid: CoA ligase) converts 4-coumaric acid to form 4-coumaroyl-CoA, which is a key enzyme in the phenylpropanoid metabolic pathway that regulates the biosynthesis of lignin and flavonoids [40].
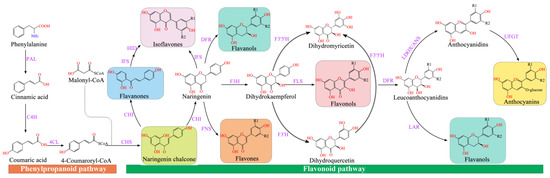
Figure 1. Flavonoid synthesis pathway. CHS (chalcone synthase) can catalyze three molecules of malonyl-CoA and one molecule of p-coumaroyl-CoA to form naringeninchalcone [41]. Malonyl-CoA is an important precursor for the synthesis of natural products, including flavonoids and polyketides [42]. CHI (chalcone isomerase) converted naringenin-chalcone into flavanones [43]. Naringenin, as an important flavonoid skeleton, is catalyzed by FNSI and FNS II (flavone synthase I and flavone synthase II) and IFS (isoflavone synthase) to form flavones and isoflavones, respectively [44]. Furthermore, flavanone-3-hydroxylase (F3H), flavonol 3′-hydroxylase (F3′H), and flavonol 3′5′-hydroxylase (F3′5′H) catalyzed naringenin to generate dihydro-myricetin, dihydro-kaempferol, and dihydro-quercetin, respectively [45]. The FLS (flavonol synthase) converted dihydroflavonols into flavonols (kaempferol, quercetin, and myricetin), which was catalyzed by the dihydroflavonol 4-reductase (DFR) to generate leucoanthocyanidins [46], which was catalyzed by leucoanthocyanidin dioxygenase (LDOX) to produce anthocyanidins [47]. Anthocyanidins and leucoanthocyanidins were further converted to proanthocyanidins catalyzed by leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR), respectively [48]. Modification of anthocyanins is responsible for the stabilization of vacuolar anthocyanins, including glycosylation, methylation, and acylation [49].
2.2. Transcriptional Regulation of Flavonoid Synthesis
Flavonoid biosynthesis is tightly regulated by biosynthetic enzymes and regulatory transcription factors (TFs) [50]. Several TF families have been reported to be involved in regulating flavonoid biosynthesis in plants, including WRKY, Dof, MADS-box, bZIP, MYB, bHLH, WD40, and NAC (Table 1) [51]. Plant MYBs are characterized by a highly conserved MYB DNA-binding domain and are further classified into four groups based on the position and number of MYB repeats: 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB [52]. Among them, R2R3-MYB TFs are involved in regulating the expression of structural genes in the flavonoid pathway [53]. For example, transgenic tobacco overexpressing NtMYB3 from Narcissus tazetta can reduce the content of flavonoids by inhibiting the expression of FLSs [54]. Transgenic Arabidopsis overexpressing GbMYB2 from Ginkgo biloba can decrease flavonoid accumulation by inhibiting the expression of some structural genes (e.g., GbPAL, GbFLS, GbANS, and GbCHI) [55]. Yan et al. revealed that soybean GmMYB100 negatively regulated flavonoid biosynthesis by inhibiting the activities of CHS and CHI promoters [56]. In addition, the overexpression of PpMYB17 in pear calli was found to bind and activate the promoters of structural genes of PpCHS, PpCHI, PpF3H, PpFLS, and PpUFGT under light conditions, which enhanced the biosynthesis of flavonoids [57]. Transgenic tobacco overexpressing FtMYB31 from Fagopyrum tataricum increased the expression of CHS, F3H, and FLS genes and promoted the accumulation of flavonoids [58]. The overexpression of SbMYB8 from Scutellaria baicalensis in transgenic tobacco promoted the expression of the SbCHS gene, increased flavonoid content, and enhanced the activities of antioxidant enzymes in transgenic tobacco [48]. Furthermore, bHLH TFs play essential roles in regulating the biosynthesis of flavonoids. CsMYC2 was able to promote flavonoid biosynthesis by increasing the expression of the UFGT gene [59]. MdbHLH3 promoted anthocyanin accumulation and fruit coloration in response to low temperatures in apples [60]. In addition, MBW complexes (MYB-bHLH-WD40) regulate flavonoid biosynthesis in different plants [49][61]. The TT2–TT8–TTG1 complex plays a major role in developing seeds and also plays an important role in regulating the expression of LBGs (DFR, LDOX, TT19, TT12, AHA10, and BAN) [62]. Moreover, the MBW complex exhibits tissue-specific regulation of the expression of the genes involved in flavonoid biosynthesis [63]. The MYB5–TT8–TTG1 complex is active in the endothelium, regulating DFR, LDOX, and TT12 expression, whereas the TT2–EGL3/GL3–TTG1 complexes regulate the expression of LDOX, BAN, AHA10, and DFR in the chalaza [63].
Table 1. Pharmacological activities of flavonoids.
| Flavonoids | Classification | Pharmacological Activity | Sources of Plant | References |
|---|---|---|---|---|
| Proanthocyanidins | anthocyanins | antioxidant, anti-inflammatory, antibacterial, antifungal and anti-cardiovascular | grapes, apples, sorghum, cherries, and other natural plant | [64] |
| Cyanidin | anthocyanins | anti-inflammatory, antiviral, and anticancer | black rice, black beans, purple potatoes, blueberries | [65] |
| Curcumin | curcuminoids | anti-inflammatory and anticancer | Curcuma longa | [66] |
| Methyl chalcone | chalcones | anti-inflammatory and anticancer | apple, citrus, soybean, ginger, mulberry | [67] |
| Trans-chalcone | chalcones | anti-inflammatory and anticancer | apple, citrus, soybean, ginger, mulberry | [67] |
| Xanthohumol | chalcones | anti-cardiovascular and antiviral | Humulus lupulus | [68] |
| Licochalcone | chalcones | antibacterial and antifungal | Glycyrrhiza uralensis | [69] |
| Catechin | flavanols | antioxidant, anti-inflammatory, antiviral, and anti-cardiovascular | Camellia sinensis | [70][71][72] |
| Epigallocatechin gallate | flavanols | antioxidant, antibacterial, antifungal, anti-cardiovascular, and antiviral | Camellia sinensis | [73][74][75] |
| Naringin | flavanones | antioxidant, anti-inflammatory, anti-cardiovascular, and antiviral | lemons, oranges, grapefruits, citrus | [67][73][76][77][78][79] |
| Hesperidin | flavanones | anti-inflammatory, anti-cardiovascular, and antiviral | lemons, limes, oranges, grapefruits, citrus | [76][77][80][81] |
| Diosmin | flavanones | anti-inflammatory | citrus fruits | [82] |
| Orientin | flavanones | anti-inflammatory | Trollius chinensis, Cajanus cajan, Crataegus laevigata | [83] |
| Vitexin | flavanones | antioxidant, anti-inflammatory, and anticancer | Ficus deltoid, Spirodela polyrhiza | [83] |
| Acacetin | flavanones | anti-cardiovascular, anticancer, and antiviral | Acacia farnesiana | [84][85] |
| Silymarin | flavanones | antioxidant, anti-cardiovascular, and antiviral | Silybum marianum | [86][87] |
| Liquiritigenin | flavanones | anti-inflammatory, antiviral, and anticancer | Glycyrrhiza uralensis | [88] |
| Isorhamnetin | flavanones | antiviral and anticancer | Ginkgo biloba, Hippophae rhamnoides | [85] |
| Apigenin | flavones | antibacterial, antifungal, and antiviral | Apium graveolens | [89][90][91][92] |
| Morin | flavones | antioxidant and anti-inflammatory | Cudrania cochinchinensis, Maclura pomifera | [93] |
| Baicalin | flavones | Anti-cardiovascular, antibacterial, and antifungal | Scutellaria baicalensis | [74][94] |
| Luteolin | flavones | anti-inflammatory, anti-cardiovascular, and antiviral | Dracocephalum integrifolium, Lonicera japonica, Capsicum annuum | [92][95] |
| Fisetin | flavonols | antioxidant | strawberry, apple, onion, cucumber, and other fruits and vegetables | [96] |
| Quercetin | flavonols | antioxidant, anti-inflammatory, anti-cardiovascular, antibacterial, and antifungal | vegetables, fruit, seeds, nuts, tea, and red wine | [71][80][97][98][99][100][101] |
| Rutin | flavonols | antioxidant, anti-inflammatory, and antiviral | rue, tobacco, jujube, apricot, orange, tomato, buckwheat, and citrus fruits | [70][80][86][87] |
| Kaempferol | flavonols | antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer | fruits, vegetables, herbs, and other natural plants | [70][93][102] |
| Myricetin | flavonols | antioxidant, anti-inflammatory, and anti-cardiovascular | Myrica rubra | [93][103][104] |
| Glabrol | isoflavane | antibacterial and antifungal | Glycyrrhiza uralensis | [69] |
| Genistein | isoflavone | antioxidant, antifungal, antiviral, and anticancer | soybeans and other plants | [80][105][106] |
In addition, several TF families, including bZIP, NAC, Dof, and WRKY, play important roles in regulating flavonoid biosynthesis [107][108]. For example, VvibZIPC22 was able to bind and activate the promoters of structural genes of VviCHI and VviCHS to increase their flavonoid contents [109]. Transgenic tobacco overexpressing NtHY5 increased the expression of phenylpropanoid pathway genes, promoted the biosynthesis of flavonoids, and enhanced plant tolerance to salt stress [110]. Transgenic Arabidopsis overexpressing AtNAC078 increased the content of flavonoids under strong light conditions by upregulating the expression of CHS, F3′H, DFR, and LDO [111]. MdNAC52 promoted the biosynthesis of flavonoid compounds (anthocyanins and procyanidins) in apples by binding and activating the promoters of MdMYB9, MdMYB11, and LAR [112]. Arabidopsis AtDOF4 upregulated the expression of structural genes of DFR, LDOX, TT19, and PAP1 to increase the content of flavonoids in plants [113]. Apple callus overexpressing MdWRKY11 was able to increase the expression of F3H, FLS, DFR, ANS, and UFGT and promote the biosynthesis of flavonoids and anthocyanins [114].
2.3. Non-Coding RNA Regulates Flavonoid Biosynthesis
Non-coding RNA, including lncRNA (long non-coding RNAs) and microRNA, played important roles in regulating flavonoid biosynthesis [115]. lncRNAs may act as precursors and endogenous target mimics of miRNAs to indirectly regulate protein-coding genes (PCgenes) [115]. Two lncRNAs, XR_001591906 and MSTRG.9304, were found to regulate the expression of the CHS gene in flavonoid biosynthesis during peanut seed development [116]. miRNAs directly cleave structural genes (SG) for flavonoid synthesis, thereby negatively regulating the accumulation of flavonoids, including miR396-targeting UFGT, miR172-targeting 4CL, and miR829.1-targeting CHS [117]. The miRNA-directed cleavage of TFs involved in flavonoid synthesis through miRNA–TF–SG regulatory networks such as miR156–SPL–F3H, miR828/TAS4–MYBs–DFR, and miR858–MYBs–CHS/FLS [117][118].
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. 2020, 60, 626–659.
- Bose, S.; Sarkar, D.; Bose, A.; Mandal, S.C. Natural flavonoids and its pharmaceutical importance. Pharma Rev. 2018, 94, 61–75.
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert. Opin. Drug Metab. Toxi. 2017, 13, 323–330.
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant compounds for plant defence and for a healthy human diet. Not. Bot. Horti Agrobot. Cluj. 2018, 46, 14–21.
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35.
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Buren, L.V.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233.
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation. Front. Plant. Sci. 2019, 10, 10.
- Ali, A.; Parisi, A.; Normanno, G. Polyphenols as emerging antimicrobial agents. In Emerging Modalities in Mitigation of Antimicrobial Resistance; Springer International Publishing: Cham, Switzerland, 2022; pp. 219–259.
- Vandercook, C.E.; Stephenson, R.G. Lemon juice composition. Identification of major phenolic compounds and estimation by paper chromatography. J. Agr. Food Chem. 1966, 14, 450–454.
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. 2022, 62, 3833–3854.
- Najmanová, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. 2020, 60, 3155–3171.
- Roy, M.; Datta, A. Fundamentals of phytochemicals. In Cancer Genetics and Therapeutics; Springer: Singapore, 2019; pp. 49–81.
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. Acad. Sci. 2012, 1259, 87–94.
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Xu, C.; Li, X.; Chen, K. Hydroxylation decoration patterns of flavonoids in horticultural crops: Chemistry, bioactivity, and biosynthesis. Hortic. Res. 2022, 9, uhab068.
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209.
- Kopečná-Zapletalová, M.; Krasulová, K.; Anzenbacher, P.; Hodek, P.; Anzenbacherová, E. Interaction of isoflavonoids with human liver microsomal cytochromes P450: Inhibition of CYP enzyme activities. Xenobiotica 2017, 47, 324–331.
- Hummelova, J.; Rondevaldova, J.; Balastikova, A.; Lapcik, O.; Kokoska, L. The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on antistaphylococcal effect of demethyltexasin. Lett. Appl. Microbiol. 2015, 60, 242–247.
- Lim, Y.J.; Jeong, H.Y.; Gil, C.S.; Kwon, S.J.; Na, J.K.; Lee, C.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food Chem. 2020, 303, 125376.
- Meng, N.; Yu, B.J.; Guo, J.S. Ameliorative effects of inoculation with Bradyrhizobium japonicum on Glycine max and Glycine soja seedlings under salt stress. Plant. Growth Regul. 2016, 80, 137–147.
- Mori-Yasumoto, K.; Hashimoto, Y.; Agatsuma, Y.; Fuchino, H.; Yasumoto, K.; Shirota, O.; Satake, M.; Sekita, S. Leishmanicidal phenolic compounds derived from Dalbergia cultrata. Nat. Prod. Res. 2021, 35, 4907–4915.
- Dutta, M.S.; Mahapatra, P.; Ghosh, A.; Basu, S. Estimation of the reducing power and electrochemical behavior of few flavonoids and polyhydroxybenzophenones substantiated by bond dissociation energy: A comparative analysis. Mol. Divers. 2022, 26, 1101–1113.
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990.
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365.
- Yang, S.; Mi, L.; Wu, J.; Liao, X.; Xu, Z. Strategy for anthocyanins production: From efficient green extraction to novel microbial biosynthesis. Crit. Rev. Food Sci. 2022, 1–16.
- Guven, H.; Arici, A.; Simsek, O. Flavonoids in our foods: A short review. J. Basic. Clin. Health Sci. 2019, 3, 96–106.
- Di Pietro, N.; Baldassarre, M.P.A.; Cichelli, A.; Pandolfi, A.; Formoso, G.; Pipino, C. Role of polyphenols and carotenoids in endothelial dysfunction: An overview from classic to innovative biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 6381380.
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Peres, L.E.P.; Zhao, J.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants–tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27.
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028.
- Kim, J.I.; Hidalgo-Shrestha, C.; Bonawitz, N.D.; Franke, R.B.; Clint, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073.
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531.
- Gonzali, S.; Perata, P. Anthocyanins from purple tomatoes as novel antioxidants to promote human health. Antioxidants 2020, 9, 1017.
- Goyal, K.; Kaur, R.; Goyal, A.; Awasthi, R. Chalcones: A review on synthesis and pharmacological activities. J. Appl. Pharm. Sci. 2021, 11, 001–014.
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic chemistry follows where nature leads. Biomolecules 2021, 11, 1203.
- Li, M.; Guo, L.; Wang, Y.; Li, Y.; Jiang, X.; Liu, Y.; Xie, D.; Gao, L.; Xia, T. Molecular and biochemical characterization of two 4-coumarate: CoA ligase genes in tea plant (Camellia sinensis). Plant Mol. Biol. 2022, 109, 579–593.
- Sun, T.; Li, S.; Song, X.; Pei, G.; Diao, J.; Cui, J.; Shi, M.; Chen, L.; Zhang, W. Re-direction of carbon flux to key precursor malonyl-CoA via artificial small RNAs in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels. 2018, 11, 1–16.
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574.
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X.; et al. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671–688.
- Cheng, A.X.; Han, X.J.; Wu, Y.F.; Lou, H.X. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095.
- Dastmalchi, M.; Dhaubhadel, S. Soybean chalcone isomerase: Evolution of the fold, and the differential expression and localization of the gene family. Planta 2015, 241, 507–523.
- Richter, A.S. Tansley insight. New Phytol. 2022, 236, 2037–2043.
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3′-hydroxylase, CsF3′ H, from Crocus sativus L: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78.
- Li, H.; Tian, J.; Yao, Y.; Zhang, J.; Song, T.; Li, K.; Yao, Y. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol. Biochem. 2019, 139, 141–151.
- Ma, S.; Hu, R.; Ma, J.; Fan, J.; Wu, F.; Wang, Y.; Huang, L.; Feng, G.; Li, D.; Nie, G.; et al. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of anthocyanins and proanthocyanidins biosynthesis in Trifolium repens. Ind. Crop. Prod. 2022, 187, 115529.
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.U.; Pate, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999.
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, Å.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240.
- Kim, M.J.; Paramanantham, A.; Lee, W.S.; Yun, J.W.; Chang, S.H.; Kim, D.C.; Park, H.S.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins Derived from Vitis coignetiae Pulliat Contributes Anti-Cancer Effects by Suppressing NF-κB Pathways in Hep3B Human Hepatocellular Carcinoma Cells and In Vivo. Molecules 2020, 25, 5445.
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458.
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471.
- Li, K.; Xing, C.; Yao, Z.; Huang, X. Pbr MYB 21, a novel MYB protein of Pyrus betulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol. J. 2017, 15, 1186–1203.
- Qin, W.; Xie, L.; Li, Y.; Liu, H.; Fu, X.; Chen, T.; Hassani, D.; Li, L.; Sun, X.; Tang, K. An R2R3-MYB transcription factor positively regulates the glandular secretory trichome initiation in Artemisia annua L. Front. Plant Sci. 2021, 12, 657156.
- Anwar, M.; Yu, W.; Yao, H.; Zhou, P.; Allan, A.C.; Zeng, L. NtMYB3, an R2R3-MYB from narcissus, regulates flavonoid biosynthesis. Int. J. Mol. Sci. 2019, 20, 5456.
- Xu, F.; Ning, Y.; Zhang, W.; Liao, Y.; Li, L.; Cheng, H.; Cheng, S. An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba. Funct. Integr. Genom. 2014, 14, 177–189.
- Yan, J.; Wang, B.; Zhong, Y.; Yao, L.; Cheng, L.; Wu, T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015, 89, 35–48.
- Premathilake, A.T.; Ni, J.; Bai, S.; Tao, R.; Teng, Y. R2R3-MYB transcription factor PpMYB17 positively regulates flavonoid biosynthesis in pear fruit. Planta 2020, 252, 1–16.
- Sun, Z.; Linghu, B.; Hou, S.; Liu, R.; Wang, L.; Hao, Y.; Han, Y.; Zhou, M.; Liu, L.; Li, H. Tartary buckwheat FtMYB31 gene encoding an R2R3-MYB transcription factor enhances flavonoid accumulation in Tobacco. J. Plant Growth Regul. 2020, 39, 564–574.
- Cultrone, A.; Cotroneo, P.S.; Reforgiato Recupero, G. Cloning and molecular characterization of R2R3-MYB and bHLH-MYC transcription factors from Citrus sinensis. Tree Genet. Genomes 2010, 6, 101–112.
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897.
- Qi, Y.; Zhou, L.; Han, L.; Zou, H.; Wang, Y. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408.
- Mano, H.; Ogasawara, F.; Sato, K.; Higo, H.; Minobe, Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 2007, 143, 1252–1268.
- Peniche-Pavía, H.A.; Guzmán, T.J.; Magaña-Cerino, J.M.; Gurrola-Díaz, C.M.; Tiessen, A. Maize Flavonoid Biosynthesis, Regulation, and Human Health Relevance: A Review. Molecules 2022, 27, 5166.
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stabil. 2017, 145, 25–40.
- Jiang, L.; Li, Z.; Xie, Y.; Liu, L.; Cao, Y. Cyanidin chloride modestly protects Caco-2 cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem. Toxicol. 2019, 127, 251–259.
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31.
- Salehi, B.; Quispe, C.; Chamkhi, I.; Omari, N.E.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharm. 2021, 11, 2068.
- Sun, T.L.; Li, W.Q.; Tong, X.L.; Liu, X.Y.; Zhou, W.H. Xanthohumol attenuates isoprenaline-induced cardiac hypertrophy and fibrosis through regulating PTEN/AKT/mTOR pathway. Eur. J. Pharmacol. 2021, 891, 173690.
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial effect and mode of action of flavonoids from licorice against methicil-lin-resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489.
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619.
- Zhang, K.; Dai, Z.; Zhang, W.; Gao, Q.; Dai, Y.; Xia, F.; Zhang, X. EDTA-based adsorbents for the removal of metal ions in wastewater. Coord. Chem. Rev. 2021, 434, 213809.
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Sig. Transduct. Target. Ther. 2021, 6, 255.
- Torello, C.O.; Alvarez, M.C.; Olalla Saad, S.T. Polyphenolic Flavonoid Compound Quercetin Effects in the Treatment of Acute Myeloid Leukemia and Myelodysplastic Syndromes. Molecules 2021, 26, 5781.
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178.
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharma. 2020, 121, 109604.
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Lagana, G.; Daglia, M.; Menehini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506.
- Chang, X.; Zhang, T.; Wang, J.; Liu, Y.; Yan, P.; Meng, Q.; Yin, Y.; Wang, S. SIRT5-related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance. Oxid. Med. Cell. Longev. 2021, 2021, 5876841.
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Mannelli, L.D.C.; Brogi, S.; et al. The citrus flavonoid naringenin protects the myocardium from ageing-dependent dysfunction: Potential role of SIRT1. Oxid. Med. Cell. Longev. 2020, 2020, 4650207.
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium Naringenin Prevents Osteosarcoma Progression and Recurrence in the Patients Who Underwent Osteosarcoma Surgery by Improving Antioxidant Capability. Oxid. Med. Cell. Longev. 2018, 7, 8713263.
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farm. 2001, 56, 683–687.
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31.
- Yang, J.; Liu, L.; Li, M.; Huang, X.; Yang, H.; Li, K. Naringenin inhibits pro-inflammatory cytokine production in macrophages through inducing MT1G to suppress the activation of NF-κB. Mol. Immunol. 2021, 137, 155–162.
- Zhong, L.; Lin, Y.; Wang, C.; Niu, B.; Xu, Y.; Zhao, G.; Zhao, J. Chemical Profile, Antimicrobial and Antioxidant Activity Assessment of the Crude Extract and Its Main Flavonoids from Tartary Buckwheat Sprouts. Molecules 2022, 27, 374.
- Wu, C.; Chen, R.L.; Wang, Y.; Wu, W.Y.; Li, G. Acacetin alleviates myocardial ischemia/reperfusion injury by inhibiting oxidative stress and apoptosis via the Nrf-2/HO-1 pathway. Pharm. Biol. 2022, 60, 553–561.
- Wu, Q.; Kroon, P.A.; Shao, H.; Needs, P. Differential effects of quercetin and two of its derivatives, isorhamnetin and isorhamnetin-3-glucuronide, in inhibiting the proliferation of human breast-cancer MCF-7 cells. J. Agr. Food Chem. 2018, 66, 7181–7189.
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Simmet, T. Chrysosplenol d, a flavonol from Artemisia annua, induces ERK1/2-mediated apoptosis in triple negative human breast cancer cells. Int. J. Mol. Sci. 2020, 21, 4090.
- Chen, X.; Wu, Y.; Gu, J.; Liang, P.; Qin, J. Anti-invasive effect and pharmacological mechanism of genistein against colorectal cancer. Biofactors 2020, 46, 620–628.
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Prétet, J.L.; Mougin, C. Isoliquiritigenin induces caspase-dependent apoptosis via downregulation of HPV16 E6 expression in cervical cancer Ca Ski cells. Planta Med. 2013, 79, 1628–1635.
- Yao, Z.; Xu, X.; Huang, Y. Daidzin inhibits growth and induces apoptosis through the JAK2/STAT3 in human cervical cancer HeLa cells. Saudi J. Biol. Sci. 2021, 28, 7077–7081.
- Michaelis, M.; Sithisarn, P.; Cinatl, J. Effects of flavonoid-induced oxidative stress on anti-H5N1 influenza a virus activity exerted by baicalein and biochanin A. BMC Res. Notes. 2014, 7, 1–6.
- Guntaka, R.V. New Insights to Prevent Liver Fibrosis by Targeting YB-1 and Collagen Genes. OBM Hepatol. Gastroenterol. 2019, 3, 1.
- Wu, J.; Zhou, T.; Wang, Y.; Jiang, Y.; Wang, Y. Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules 2021, 26, 5949.
- Figueiredo-Rinhel, A.S.; Santos, E.O.; Kabeya, L.M.; Azzolini, A.E.; Simões-Ambrosio, L.M.; Lucisano-Valim, Y.M. The flavonols quercetin, myricetin, kaempferol, and galangin inhibit the net oxygen consumption by immune complex-stimulated human and rabbit neutrophils. Z. Nat. C J. Biosci. 2014, 69, 346–356.
- Farkhondeh, T.S.; Samarghandian Bafandeh, F. The Cardiovascular Protective Effects of Chrysin: A Narrative Review on Experimental Researches. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 17–27.
- Khazeei Tabari, M.A.; Iranpanah, A.; Bahramsoltani, R.; Rahimi, R. Flavonoids as promising antiviral agents against SARS-CoV-2 infection: A mechanistic review. Molecules 2021, 26, 3900.
- Zeng, N.; Zhang, G.; Hu, X.; Pan, J.; Gong, D. Mechanism of fisetin suppressing superoxide anion and xanthine oxidase activity. J. Funct. Foods 2019, 58, 1–10.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75.
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23.
- Sugawara, T.; Sakamoto, K. Quercetin enhances motility in aged and heat-stressed Caenorhabditis elegans nematodes by modulating both HSF-1 activity, and insulin-like and p38-MAPK signalling. PLoS ONE 2020, 15, e0238528.
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Devi, K.P.; Tejada, S.; Sureda, A.; Xu, S.W.; Yousefi, B.; Majidinia, M.; et al. Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway. Pharma. Res. 2020, 152, 104626.
- Kiani, K.; Dhuli, K.; Anpilogov, K.; Bressan, S.; Dautaj, A.; Dundar, M.; Beccari, T.; Ergoren, M.; Bertelli, M. Natural compounds as inhibitors of SARS-CoV-2 endocytosis: A promising approach against COVID-19. Acta Med. 2020, 91, 13.
- Kandhari, K.; Mishra, J.P.N.; Singh, R.P. Acacetin inhibits cell proliferation, survival, and migration in human breast cancer cells. Int. J. Pharma. Biol. Sci. 2019, 9, 443–452.
- Zhou, B.; Fang, L.; Dong, Y.; Yang, J.; Chen, X.; Zhang, N.; Zhu, Y.; Huang, T. Mitochondrial quality control protects photoreceptors against oxidative stress in the H2O2-induced models of retinal degeneration diseases. Cell Death Dis. 2021, 12, 413.
- Wang, M.; Liu, Y.; Pan, R.L.; Wang, R.Y.; Ding, S.L.; Dong, W.R.; Sun, X.B. Protective effects of Myrica rubra flavonoids against hypoxia/reoxygenation-induced cardiomyocyte injury via the regulation of the PI3K/Akt/GSK3β pathway. Int. J. Mol. Med. 2019, 43, 2133–2143.
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharm. 2019, 117, 109086.
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65.
- Qian, Y.; Zhang, T.; Yu, Y.; Gou, L.; Pi, E. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021, 12, 1143.
- Qiu, Z.; Wang, H.; Li, D.; Byu, B.; Cao, B. Identification of Candidate HY5-Dependent and-Independent Regulators of Anthocyanin Biosynthesis in Tomato. Plant Cell Physiol. 2019, 60, 643–656.
- Malacarne, G.; Coller, E.; Czemmel, S.; Vrhovsek, U.; Engelen, K.; Goremykin, V.; Bogs, J.; Moser, C. The grapevine VvibZIPC22 transcription factor is involved in the regulation of flavonoid biosynthesis. J. Exp. Bot. 2016, 67, 3509–3522.
- Singh, D.; Singh, H.; Singh, N.; Dwivedi, S.; Trivedi, P.K. Tobacco HY5, NtHY5, positively regulates flavonoid biosynthesis and enhances salt stress tolerance. bioRxiv 2022.
- Morishita, T.; Kojima, Y.; Maruta, T.; Nishizawa-Yokoi, A.; Yabuta, Y.; Shigeoka, S. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol. 2009, 50, 2210–2222.
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662.
- Skirycz, A.; Jozefczuk, S.; Stobiecki, M.; Muth, D.; Zanor, M.I.; Witt, I.; Mueller-Roeber, B. Transcription factor AtDOF4; 2 affects phenylpropanoid metabolism in Arabidopsis thaliana. New Phytol. 2007, 175, 425–438.
- Wang, N.; Liu, W.; Zhang, T.; Jiang, S.; Xu, H.; Wang, Y.; Zhang, Z.; Wang, C.; Chen, X. Transcriptomic analysis of red-fleshed apples reveals the novel role of MdWRKY11 in flavonoid and anthocyanin biosynthesis. J. Agr. Food Chem. 2018, 66, 7076–7086.
- Liu, S.; Wang, L.; Cao, M.; Pang, S.; Wang, L. Identification and characterization of long non-coding RNAs regulating flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crop. Prod. 2020, 158, 112980.
- Ma, X.; Zhang, X.; Traore, S.M.; Xin, Z.; Yin, D. Genome-wide identification and analysis of long noncoding RNAs (lncRNAs) during seed development in peanut (Arachis hypogaea L.). BMC Plant Biol. 2020, 20, 1–14.
- Gupta, O.P.; Karkute, S.G.; Banerjee, S.; Meena, N.L.; Dahuja, A. Contemporary understanding of miRNA-based regulation of secondary metabolites biosynthesis in plants. Front. Plant Sci. 2017, 8, 374.
- Sharma, D.; Tiwari, M.; Pandey, A.; Bhatia, C.; Sharma, A.; Trivedi, P.K. MicroRNA858 is a potential regulator of phenylpropanoid pathway and plant development. Plant Physiol. 2016, 171, 944–959.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.1K
Revisions:
2 times
(View History)
Update Date:
04 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No