Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Shen Chen and Version 2 by Jessie Wu.
Flavonoids are mainly found in plant cell vacuoles in the form of C-glycosides or O-glycosides. The basic molecular structure of flavonoids depends upon their basic C6–C3–C6 skeleton. Flavonoids are classified into seven subclasses based on modifications to their basic skeletons; these subclasses include flavones, flavanones, isoflavones, flavonols, chalcones, flavanols, and anthocyanins.
- flavonoids
- biosynthesis pathway
- classification
- biological activity
- application
1. Flavonoid Classification
1.1. Flavones
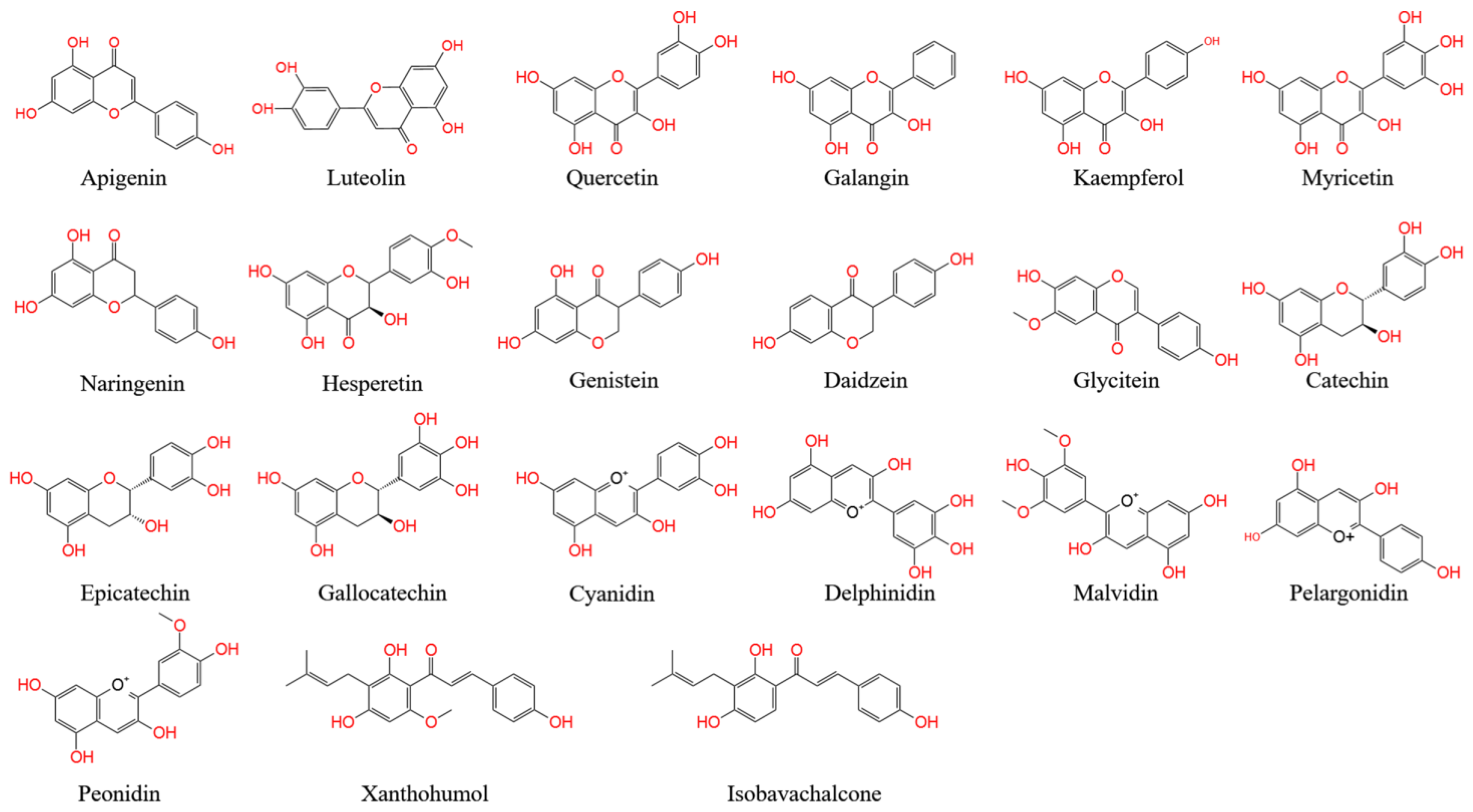
Flavones, one of the largest classes of flavonoids, consist of 4H-chromen-4-one bearing a phenyl substituent at position 2. Flavones mostly occur as 7-O-glycosides, which are found in celery, parsley, red pepper, chamomile, mint, and ginkgo [1][2][3][5,19,20]. Apigenin and luteolin are two common flavones (Figure A1). In nature, apigenin is usually found in a glycosylated form, with a sugar moiety attached to the tricyclic core structure via hydroxyl groups (O-glycosides) or directly to carbon (C-glycosides) [4][21]. The principal ingredients of apigenin are glycosylated apiin, apigenin, vitexin, isovitexin, or rhoifolin. Apigenin can scavenge free radicals and regulate antioxidant enzyme activity in pancreatic cells, and apigenin can decrease inflammation in cancer, neuroinflammation, and cardiovascular diseases [5][6][9,22].
1.2. Flavonols
Flavonols, also called 3-hydroxy flavone, can be identified by specific substitutions in their A- and B-rings, which are connected by a three-carbon chain [7][23]. Flavonols possess hydroxyl groups at positions 5 and 7 in the A-ring and are mainly present in epidermal cells to protect DNA against UV-induced damage [8][24]. Four types of flavonol compounds (quercetin, galangin, kaempferol, and myricetin) are mainly distributed in vegetables and fruits, such as asparagus, onions, lettuce, broccoli, tomato, and apples (Figure A1) [9][25]. Flavonols exhibit interesting biological activities, including antioxidant, antibacterial, cardioprotective, anticancer, and antiviral activities. Dietary flavonols can significantly decrease the risk of gastric cancer in smokers and in women (Figure A1).
1.3. Flavanones
Flavanones (dihydro-flavones) possess a saturated C-ring [10][26]. The saturated double bond between positions 2 and 3 in the C-ring represents the only structural difference between flavanones and other flavonoid compounds [11][27]. Flavanones are mainly distributed in citrus fruits, including oranges, lemons, mandarins, grapefruits, clementines, and limes [12][28]. Flavanones contain hydroxyl groups at positions 5 and 7 in the A-ring and possess hydroxyl/methoxy substituents at the C3 or C4 positions of the B-ring [13][29]. The defining characteristic of flavanones is a disaccharidic moiety linked to the seven positions of aglycone [14][30]. Depending on their structural differences, flavanones can occur in the form of naringin, naringenin, hesperidin, hesperetin, pinocembrin, likvirtin, and eriodictyol [15][31]. Among them, naringenin and hesperetin, as the main dietary flavanones, occur almost exclusively in citrus fruits (Figure A12) [12][16][28,32]. Naringin can increase the activity of antioxidant enzymes (CAT, PON, GPx, and SOD) and enhance the immune system. Furthermore, naringenin and hesperetin have been shown to recover impaired thyroid function in rats.
1.4. Isoflavonoids
Isoflavones have a B-ring at the C3 position of the heterocyclic C-ring of the diphenylpropane (C6–C3–C6) backbone, which represents their only chemical structural difference from other flavonoids [17][33]. Isoflavonoids are characteristic metabolites of leguminous plants and play essential roles in nodule induction and microbial signaling in legumes [18][19][34,35]. Isoflavones are classified into three groups: genistein, daidzein, and glycitein (Figure A1) [20][36]. The molecular structure of isoflavones is similar to that of animal estrogens. Isoflavones are phytoestrogens that exhibit potent estrogenic activity. Phytoestrogens are similar in structure to the human female hormone 17-β-estradiol in that they bind to estrogen receptors [21][37]. In addition, isoflavones possess a strong antioxidant activity, which can decrease the risk of cancers by inhibiting free radical-induced DNA damage [21][37].
1.5. Flavanols
Flavanols, also called catechins or flavan-3-ols, are characterized by a hydroxyl group at position 3 in the C-ring [22][38]. Flavanols lack a double bond between positions 2 and 3 in the C-ring [23][39]. Several flavanols, including catechin, gallocatechin 3-gallate, gallocatechin, epicatechin, epicatechin 3-gallate, catechin 3-gallate, and epicatechin 3-gallate, are widely distributed in many fruits (e.g., apples, bananas, pears, and blueberries) [24][25][40,41]. Flavanols can protect blood vessels against tobacco by increasing the content of NO in blood vessels. A flavanol-rich diet can facilitate the permanent improvement of endothelial function and prevent the development of cardiovascular diseases [26][27][42,43].
1.6. Anthocyanins
Anthocyanins, as glycosylated polyphenolic compounds, are a group of soluble vacuolar pigments that possess a range of colors, from orange, red, and purple to blue, depending on the pH of the micro-environment of the flowers, seeds, fruits, and vegetative tissues [28][44]. The position and number of hydroxyl and methoxyl groups present as substituents in the flavylium structure result in different anthocyanins (Figure A1). Thus, over 650 anthocyanins have been identified in many plants [29][45]; these are grouped into five items, including cyanidin, delphinidin, malvidin, pelargonidin, and peonidin, and their corresponding derivatives [30][46]. Anthocyanins are mainly found in the outer cell layer of various fruits and vegetables, such as blackcurrants, grapes, and berries [31][32][47,48]. The antioxidant ability of anthocyanins is associated with their ring orientation and the position and number of free hydroxyls around the pyrone ring. Anthocyanins play important roles in visual acuity, cholesterol decomposition, and the reduced risk of cardiovascular disease in humans [33][34][49,50]. In addition, anthocyanins are commonly used as food colorants.
1.7. Chalcones
Chalcones (1,3-diaryl-2-propen-1-ones) are natural open-chain flavonoids, carrying up to three modified or unmodified C5-, C10-, and C15-prenyl moieties on both their A and B-rings. These bioactive products are widely distributed in the Fabaceae, Moraceae, Zingiberaceae, and Cannabaceae families [5][9]. They exhibit a wide spectrum of pharmacological effects, including antioxidant, antibacterial, anthelmintic, antiulcer, antiviral, antiprotozoal, and anticancer effects [35][51]. Chalcones are precursors of flavonoids and isoflavonoids. Their structural features are easily constructed from simple aromatic compounds. Their prominent bioactivity has inspired the synthesis of chalcone analogs, as well as minor structural modifications to natural chalcones; these compounds form a large collection of bioactive chalcone derivatives [36][52]. Xanthohumol and isbavirachalone are two representative derivatives that exhibit abundant biological and pharmacological activity (Figure A1) [33][49].
Generally, the number and position of –OH groups have a great influence on flavonoid bioactivity. The –OH groups can link to the carbon atoms of the benzene ring (3,5,7, and 3′,4′-dihydroxy substitution pattern), which directly determines the bioactivity of flavonoids. Moreover, the position of the –OH group also influenced the flavonoid bioactivity. The most effective radical scavengers are flavonoids with the 3′,4′-dihydroxy substitution pattern on the B-ring and/or hydroxyl group at the C-3 position. In addition, the C2–C3 double bond is not necessary for high activity. Flavanols lacking the C2–C3 double bond displayed strong activity. The presence of a 3 –OH group significantly enhances the bioactivity.
2. Flavonoid Biosynthesis in Plants
2.1. Flavonoid Biosynthetic Pathways
Flavonoid synthesis occurs at the junction of the shikimate pathway and the acetate pathway. The former can generate p-coumaroyl-CoA, and the latter regulates C2-chain elongation [37][53] (Figure 1). Phenylalanine ammonia-lyase (PAL) deaminates phenylalanine to ammonia and cinnamic acid [38][54]. Then, C4H (cinnamic acid 4-hydroxylase) catalyzes the production of 4-coumaric acid [39][4], and 4CL (4-coumaric acid: CoA ligase) converts 4-coumaric acid to form 4-coumaroyl-CoA, which is a key enzyme in the phenylpropanoid metabolic pathway that regulates the biosynthesis of lignin and flavonoids [40][55].
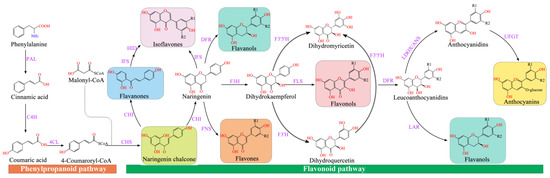
Figure 1. Flavonoid synthesis pathway. CHS (chalcone synthase) can catalyze three molecules of malonyl-CoA and one molecule of p-coumaroyl-CoA to form naringeninchalcone [41][56]. Malonyl-CoA is an important precursor for the synthesis of natural products, including flavonoids and polyketides [42][57]. CHI (chalcone isomerase) converted naringenin-chalcone into flavanones [43][58]. Naringenin, as an important flavonoid skeleton, is catalyzed by FNSI and FNS II (flavone synthase I and flavone synthase II) and IFS (isoflavone synthase) to form flavones and isoflavones, respectively [44][59]. Furthermore, flavanone-3-hydroxylase (F3H), flavonol 3′-hydroxylase (F3′H), and flavonol 3′5′-hydroxylase (F3′5′H) catalyzed naringenin to generate dihydro-myricetin, dihydro-kaempferol, and dihydro-quercetin, respectively [45][60]. The FLS (flavonol synthase) converted dihydroflavonols into flavonols (kaempferol, quercetin, and myricetin), which was catalyzed by the dihydroflavonol 4-reductase (DFR) to generate leucoanthocyanidins [46][61], which was catalyzed by leucoanthocyanidin dioxygenase (LDOX) to produce anthocyanidins [47][62]. Anthocyanidins and leucoanthocyanidins were further converted to proanthocyanidins catalyzed by leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR), respectively [48][63]. Modification of anthocyanins is responsible for the stabilization of vacuolar anthocyanins, including glycosylation, methylation, and acylation [49][64].
2.2. Transcriptional Regulation of Flavonoid Synthesis
Flavonoid biosynthesis is tightly regulated by biosynthetic enzymes and regulatory transcription factors (TFs) [50][65]. Several TF families have been reported to be involved in regulating flavonoid biosynthesis in plants, including WRKY, Dof, MADS-box, bZIP, MYB, bHLH, WD40, and NAC (Table 1) [51][66]. Plant MYBs are characterized by a highly conserved MYB DNA-binding domain and are further classified into four groups based on the position and number of MYB repeats: 1R-MYB, 2R-MYB, 3R-MYB, and 4R-MYB [52][67]. Among them, R2R3-MYB TFs are involved in regulating the expression of structural genes in the flavonoid pathway [53][68]. For example, transgenic tobacco overexpressing NtMYB3 from Narcissus tazetta can reduce the content of flavonoids by inhibiting the expression of FLSs [54][69]. Transgenic Arabidopsis overexpressing GbMYB2 from Ginkgo biloba can decrease flavonoid accumulation by inhibiting the expression of some structural genes (e.g., GbPAL, GbFLS, GbANS, and GbCHI) [55][70]. Yan et al. revealed that soybean GmMYB100 negatively regulated flavonoid biosynthesis by inhibiting the activities of CHS and CHI promoters [56][71]. In addition, the overexpression of PpMYB17 in pear calli was found to bind and activate the promoters of structural genes of PpCHS, PpCHI, PpF3H, PpFLS, and PpUFGT under light conditions, which enhanced the biosynthesis of flavonoids [57][72]. Transgenic tobacco overexpressing FtMYB31 from Fagopyrum tataricum increased the expression of CHS, F3H, and FLS genes and promoted the accumulation of flavonoids [58][73]. The overexpression of SbMYB8 from Scutellaria baicalensis in transgenic tobacco promoted the expression of the SbCHS gene, increased flavonoid content, and enhanced the activities of antioxidant enzymes in transgenic tobacco [48][63]. Furthermore, bHLH TFs play essential roles in regulating the biosynthesis of flavonoids. CsMYC2 was able to promote flavonoid biosynthesis by increasing the expression of the UFGT gene [59][74]. MdbHLH3 promoted anthocyanin accumulation and fruit coloration in response to low temperatures in apples [60][75]. In addition, MBW complexes (MYB-bHLH-WD40) regulate flavonoid biosynthesis in different plants [49][61][64,76]. The TT2–TT8–TTG1 complex plays a major role in developing seeds and also plays an important role in regulating the expression of LBGs (DFR, LDOX, TT19, TT12, AHA10, and BAN) [62][77]. Moreover, the MBW complex exhibits tissue-specific regulation of the expression of the genes involved in flavonoid biosynthesis [63][78]. The MYB5–TT8–TTG1 complex is active in the endothelium, regulating DFR, LDOX, and TT12 expression, whereas the TT2–EGL3/GL3–TTG1 complexes regulate the expression of LDOX, BAN, AHA10, and DFR in the chalaza [63][78].
Table 1.
Pharmacological activities of flavonoids.
| Flavonoids | Classification | Pharmacological Activity | Sources of Plant | References |
|---|---|---|---|---|
| Proanthocyanidins | anthocyanins | antioxidant, anti-inflammatory, antibacterial, antifungal and anti-cardiovascular | grapes, apples, sorghum, cherries, and other natural plant | [64][93] |
| Cyanidin | anthocyanins | anti-inflammatory, antiviral, and anticancer | black rice, black beans, purple potatoes, blueberries | [65][108] |
| Curcumin | curcuminoids | anti-inflammatory and anticancer | Curcuma longa | [66][109] |
| Methyl chalcone | chalcones | anti-inflammatory and anticancer | apple, citrus, soybean, ginger, mulberry | [67][110] |
| Trans-chalcone | chalcones | anti-inflammatory and anticancer | apple, citrus, soybean, ginger, mulberry | [67][110] |
| Xanthohumol | chalcones | anti-cardiovascular and antiviral | Humulus lupulus | [68][111] |
| Licochalcone | chalcones | antibacterial and antifungal | Glycyrrhiza uralensis | [69][112] |
| Catechin | flavanols | antioxidant, anti-inflammatory, antiviral, and anti-cardiovascular | Camellia sinensis | [70][71][72][101,102,113] |
| Epigallocatechin gallate | flavanols | antioxidant, antibacterial, antifungal, anti-cardiovascular, and antiviral | Camellia sinensis | [73][74][75][104,114,115] |
| Naringin | flavanones | antioxidant, anti-inflammatory, anti-cardiovascular, and antiviral | lemons, oranges, grapefruits, citrus | [67][73][76][77][78][79][104,110,116,117,118,119] |
| Hesperidin | flavanones | anti-inflammatory, anti-cardiovascular, and antiviral | lemons, limes, oranges, grapefruits, citrus | [76]][116[77],117[80][81,120,121] |
| Diosmin | flavanones | anti-inflammatory | citrus fruits | [82][122] |
| Orientin | flavanones | anti-inflammatory | Trollius chinensis, Cajanus cajan, Crataegus laevigata | [83][123] |
| Vitexin | flavanones | antioxidant, anti-inflammatory, and anticancer | Ficus deltoid, Spirodela polyrhiza | [83][123] |
| Acacetin | flavanones | anti-cardiovascular, anticancer, and antiviral | Acacia farnesiana | [84][85][124,125] |
| Silymarin | flavanones | antioxidant, anti-cardiovascular, and antiviral | Silybum marianum | [86][126[87],127] |
| Liquiritigenin | flavanones | anti-inflammatory, antiviral, and anticancer | Glycyrrhiza uralensis | [88][128] |
| Isorhamnetin | flavanones | antiviral and anticancer | Ginkgo biloba, Hippophae rhamnoides | [85][125] |
| Apigenin | flavones | antibacterial, antifungal, and antiviral | Apium graveolens | [89][90][91][92][129,130,131,132] |
| Morin | flavones | antioxidant and anti-inflammatory | Cudrania cochinchinensis, Maclura pomifera | [93][133] |
| Baicalin | flavones | Anti-cardiovascular, antibacterial, and antifungal | Scutellaria baicalensis | [74][94][114,134] |
| Luteolin | flavones | anti-inflammatory, anti-cardiovascular, and antiviral | Dracocephalum integrifolium, Lonicera japonica, Capsicum annuum | [92][95][132,135] |
| Fisetin | flavonols | antioxidant | strawberry, apple, onion, cucumber, and other fruits and vegetables | [96] |
| Quercetin | flavonols | antioxidant, anti-inflammatory, anti-cardiovascular, antibacterial, and antifungal | vegetables, fruit, seeds, nuts, tea, and red wine | [71][80][97][98][99][100][101][100,102,107,120,136,137,138] |
| Rutin | flavonols | antioxidant, anti-inflammatory, and antiviral | rue, tobacco, jujube, apricot, orange, tomato, buckwheat, and citrus fruits | [70][80][86][87][101,120,126,127] |
| Kaempferol | flavonols | antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer | fruits, vegetables, herbs, and other natural plants | [70][93][102][101,133,139] |
| Myricetin | flavonols | antioxidant, anti-inflammatory, and anti-cardiovascular | Myrica rubra | [93][103][104][133,140,141] |
| Glabrol | isoflavane | antibacterial and antifungal | Glycyrrhiza uralensis | [69][112] |
| Genistein | isoflavone | antioxidant, antifungal, antiviral, and anticancer | soybeans and other plants | [80][105][106][120,142,143] |
In addition, several TF families, including bZIP, NAC, Dof, and WRKY, play important roles in regulating flavonoid biosynthesis [107][108][79,80]. For example, VvibZIPC22 was able to bind and activate the promoters of structural genes of VviCHI and VviCHS to increase their flavonoid contents [109][81]. Transgenic tobacco overexpressing NtHY5 increased the expression of phenylpropanoid pathway genes, promoted the biosynthesis of flavonoids, and enhanced plant tolerance to salt stress [110][82]. Transgenic Arabidopsis overexpressing AtNAC078 increased the content of flavonoids under strong light conditions by upregulating the expression of CHS, F3′H, DFR, and LDO [111][83]. MdNAC52 promoted the biosynthesis of flavonoid compounds (anthocyanins and procyanidins) in apples by binding and activating the promoters of MdMYB9, MdMYB11, and LAR [112][84]. Arabidopsis AtDOF4 upregulated the expression of structural genes of DFR, LDOX, TT19, and PAP1 to increase the content of flavonoids in plants [113][85]. Apple callus overexpressing MdWRKY11 was able to increase the expression of F3H, FLS, DFR, ANS, and UFGT and promote the biosynthesis of flavonoids and anthocyanins [114][86].
2.3. Non-Coding RNA Regulates Flavonoid Biosynthesis
Non-coding RNA, including lncRNA (long non-coding RNAs) and microRNA, played important roles in regulating flavonoid biosynthesis [115][87]. lncRNAs may act as precursors and endogenous target mimics of miRNAs to indirectly regulate protein-coding genes (PCgenes) [115][87]. Two lncRNAs, XR_001591906 and MSTRG.9304, were found to regulate the expression of the CHS gene in flavonoid biosynthesis during peanut seed development [116][88]. miRNAs directly cleave structural genes (SG) for flavonoid synthesis, thereby negatively regulating the accumulation of flavonoids, including miR396-targeting UFGT, miR172-targeting 4CL, and miR829.1-targeting CHS [117][89]. The miRNA-directed cleavage of TFs involved in flavonoid synthesis through miRNA–TF–SG regulatory networks such as miR156–SPL–F3H, miR828/TAS4–MYBs–DFR, and miR858–MYBs–CHS/FLS [117][118][89,90].