Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farisha Alia Norfuad | -- | 2757 | 2023-06-29 17:37:47 | | | |
| 2 | Rita Xu | Meta information modification | 2757 | 2023-06-30 03:42:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Norfuad, F.A.; Mokhtar, M.H.; Nur Azurah, A.G. Probiotics on Benign Gynaecological Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/46232 (accessed on 07 February 2026).
Norfuad FA, Mokhtar MH, Nur Azurah AG. Probiotics on Benign Gynaecological Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/46232. Accessed February 07, 2026.
Norfuad, Farisha Alia, Mohd Helmy Mokhtar, Abdul Ghani Nur Azurah. "Probiotics on Benign Gynaecological Disorders" Encyclopedia, https://encyclopedia.pub/entry/46232 (accessed February 07, 2026).
Norfuad, F.A., Mokhtar, M.H., & Nur Azurah, A.G. (2023, June 29). Probiotics on Benign Gynaecological Disorders. In Encyclopedia. https://encyclopedia.pub/entry/46232
Norfuad, Farisha Alia, et al. "Probiotics on Benign Gynaecological Disorders." Encyclopedia. Web. 29 June, 2023.
Copy Citation
Probiotics are live microorganisms that confer beneficial effects on human health when an adequate dose is administered. The use of probiotics has gained tremendous interest from the public due to its promising effects in the management of various reproductive diseases.
probiotics
vaginal infection
polycystic ovary syndrome
1. Introduction
Probiotics are defined as “living microorganisms that, when administered in sufficient amounts, confer health benefits to the host” [1]. It is important to note that probiotics is a broad term that refers to various microbes, characterised by their genus, species and strain names [2]. Today, strains classified as lactic acid bacteria are the most important in food and nutrition, and strains belonging to the genera Lactobacillus and Bifidobacterium are the most commonly used probiotics, which have essential properties in a practical context [3]. Other examples of probiotics from other genera include Bacillus, Streptococcus, Enterococcus, Saccharomyces and Escherichia coli (Table 1).
Table 1. Microorganisms that are considered probiotics.
| Microorganisms Considered Probiotics | |||
|---|---|---|---|
| Lactobacillus Species | Bifidobacterium Species | Other Lactic Acid Bacteria | Non-Lactic Acid Bacteria |
| L. acidophilus | B. adolescentis | Enterococcus faecalis | Bacillus cereus var. toyoi |
| L. amylovorus | B. animalis | Enterococcus faecium | Escherichia coli strain nissle |
| L. casei | B. bifidum | Lactococcus lactis | Propionibacterium freudenreichii |
| L. crispatus | B. breve | Leuconstoc mesenteroides | Saccharomyces cerevisiae |
| L. delbrueckii | B. infantis | Pedioccocus acidilactic | Saccharomyces boulardii |
| L. gallinarum | B. lactis | Sprolactobacillus inulinus | |
| L. gasseri | B. longum | Streprococcus thermophilus | |
| L. johnsonii | |||
| L. paracasei | |||
| L. reuteri | |||
| L. rhamnosus | |||
A potential probiotic must possess desirable properties to exert its beneficial effects (Figure 1). According to Fuller (1989) [4], an ideal probiotic must possess certain characteristics: (1) It should be a strain that is capable of having a positive effect on the host, such as greater growth or disease resistance; (2) It should be exist in the form of living cells, preferably in large numbers, and it should be safe, non-invasive, non-pathogenic and non-toxic; (3) It should be able to survive and be resilient in the gut environment; (4) It should be stable and able to have long-term viability, i.e., survive for long periods in storage or in the field [4]. Kechagia et al. (2012) also stated that a probiotic should be of human origin, be able to adhere effectively to human intestinal cells and mucins to successfully modulate the immune system, possess antimicrobial properties against pathogenic bacteria and be microbiologically characterised. In addition, they must be tested in randomised clinical trials [5].
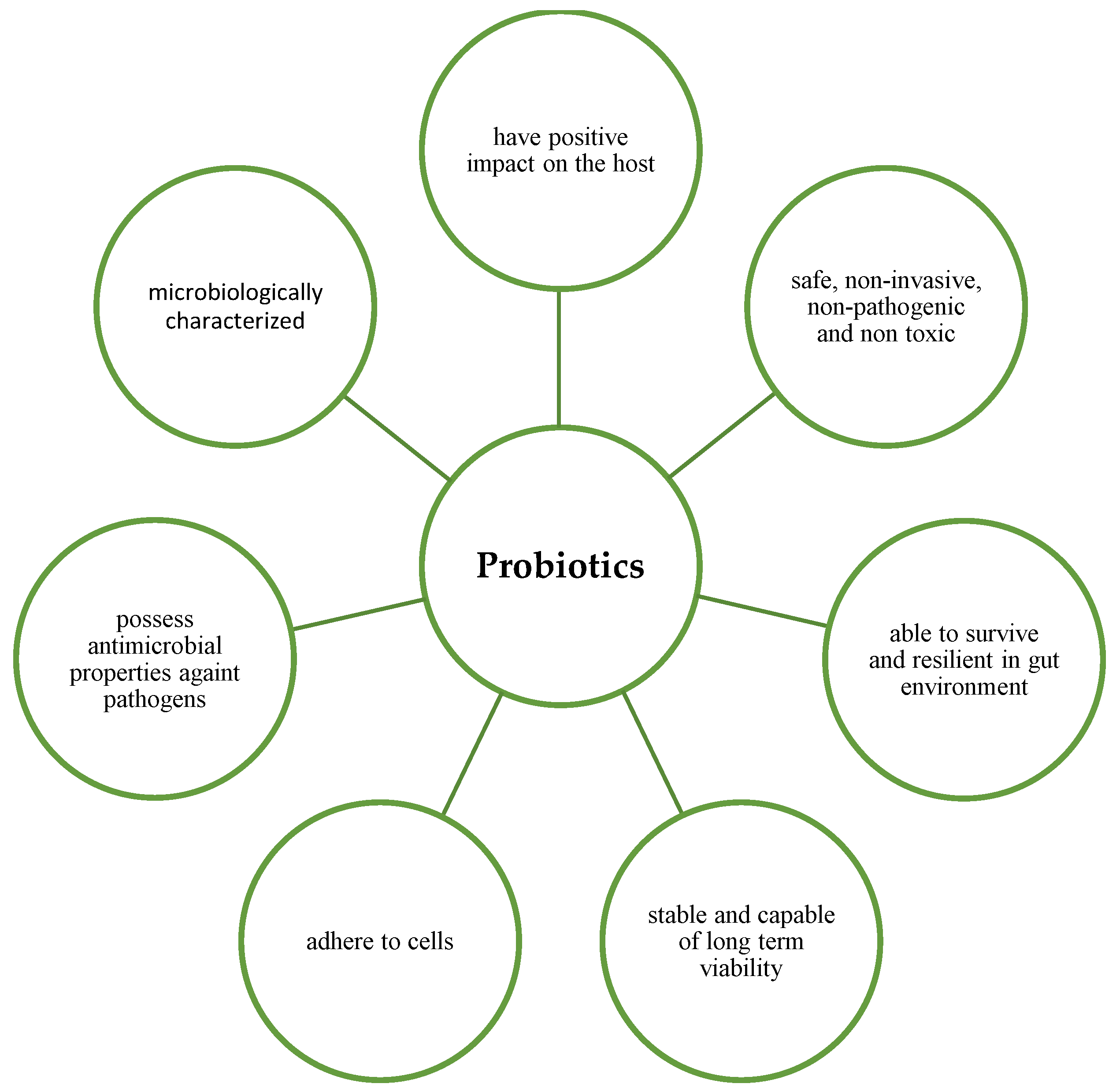
Figure 1. Characteristics of ideal probiotics.
The sufficient dosage of probiotic microorganisms required to achieve beneficial health effects depends on the strain and the product [6]. In general, products containing probiotics should have a minimum number of viable cells with proven efficacy based on human clinical trials to transfer the effect of probiotics to the consumer. This is estimated to be between 106 and 108 colony-forming units per gramme (CFU/g) of the final product or 108 to 1010 CFU/day (taking into account 100 g or 100 mL of ingested food) [7]. The rationale for administrating oral probiotics for the treatment of gynaecological disorders is related to the ability of the microorganisms to survive the gastrointestinal tract and to ascend the vaginal tract after excretion from the rectum [8]. In contrast, vaginal administration allows a direct and targeted colonisation action of probiotics to restore the balance of a normal vaginal microflora [7].
Numerous studies have shown the positive effects of probiotics on gynaecological health in humans [9][10][11][12][13][14][15][16][17][18][19][20][21][22] and animal models [23][24]. In humans, the consumption of probiotics has been shown to be beneficial for the female reproductive system. When administered orally or vaginally to women, they are effective against gynaecological disorders, such as vaginosis, polycystic ovary syndrome, and improve the gut microbiota [25]. The administration of probiotics has been proposed as a therapeutic approach to prevent these gynaecological diseases. Based on this information, it is now believed that there is an interaction between the vaginal microbiota, probiotics, and gynaecological health and diseases (Figure 2).
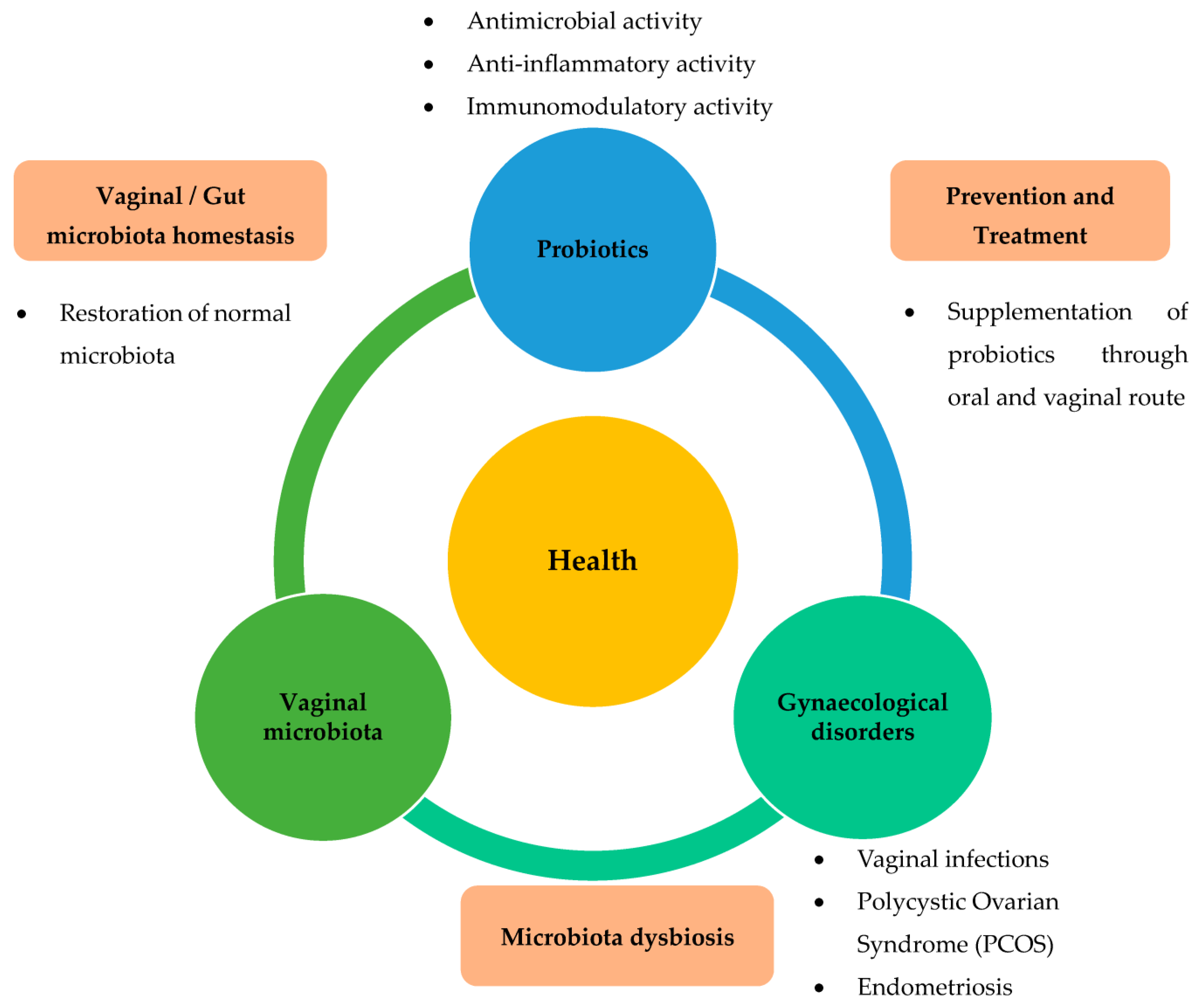
Figure 2. Role of probiotics relevant to gynaecological disorders via restoration of dysbiosis in the vaginal or gut microbiota. Various gynaecological disorders, such as vaginal infections, PCOS, and endometriosis, are known to be closely related to dysbiosis of vaginal or gut microbiota. Administration of probiotics has been suggested as a therapeutic approach to prevent these gynaecological diseases. Based on this information, it is now believed that there is an interplay between vaginal microbiota, probiotics, and gynaecological health and diseases.
2. Beneficial Effects of Probiotics on Benign Gynaecological Disorders
2.1. Effects of Probiotics on Vaginal Infections
To date, vaginal infections are some of the most common reasons for gynaecological consultation in women of childbearing age. The most diagnosed condition is bacterial vaginosis (BV), apart from other infections, including candidiasis and trichomoniasis [26]. BV indicates aberrant alterations in the normal vaginal microbiota, due to the depletion or increased concentrations of the dominant lactic acid-producing Lactobacilli spp., as well as excessive proliferation of aerobic or anaerobic bacteria, normally found in the vagina [27]. BV also contributes to an increase in the local levels of pro-inflammatory cytokines and damages the epithelial and mucosal barrier. Hence, if left untreated, even the usually mild and asymptomatic BV infection can lead to an increased risk of more severe gynaecological disorders, such as endometritis, pelvic inflammatory disease, chronic vaginitis and infertility [28].
Probiotic therapies are emerging as popular treatment for BV. Lactobacillus spp. is the genus with the most well-known intravaginal beneficial probiotic species, which breaks down carbohydrates and maintains an acidic intravaginal microflora by producing lactic acid and carbon dioxide, thus preventing the growth of pathogenic microbes, such as Enterobacteria, Escherichia coli, Candida spp., and G. vaginalis, from colonising the vaginal canal [10][26][27]. Several vaginal Lactobacillus spp. also have protective characteristics, including the production of hydrogen peroxide and their ability to colonise the vaginal tract and adhere to the vaginal epithelial cells [29]. Apart from the characteristics of the strain, most clinical trials with positive results used probiotic formulations with high doses of Lactobacilli spp., about 109 CFU, showing that the dosage of the administered probiotics may play a role in the success of the treatment [10][11][12][13][30][31].
Currently, the main pathogenic mechanism in BV is thought to be the loss of lactic acid-producing Lactobacilli spp. and the overgrowth of BV associated anaerobes such as Gardnerella spp., Atopobium spp., Prevotella spp., and Mobiluncus spp. among the vaginal microbiota [7][13]. Under these pathogenic conditions, the vaginal pH fails to be maintained at the normal range of 3.8–4.5 due to the low lactic acid level, and the hydrolytic enzymes (such as sialidase and prolidase) demolish the normal vaginal barrier, which elicits an increase in immune responses, including the release of pro-inflammatory cytokines and chemokines, such as IL-6, IL-8, IL-1α, IL-1β, TNF-α, and many others [7][32]. The synergistic interactions between pathogenic microbes increase the bacterial load and enhance the severity of BV.
Restoration of the vaginal microbiota by administration of probiotics was reported in five studies [9][12][14][32]. Ya et al. [9] recruited 120 healthy women of reproductive age with a history of BV and subjected them to BV prophylaxis with a proprietary vaginal probiotic capsule, Probaclac Vaginal, containing 108 CFU of live lactic bacteria, including L. rhamnosus, L. acidophilus, Streprococcus thermophilus and lactose for 2 months. Throughout the treatment period, probiotic prophylaxis was reported to be successful in reducing discharge, lowering vaginal pH and dramatically reducing BV recurrence (15.8% vs. 45.0%; p < 0.001) and G. vaginalis risk in women with a history of recurrent BV. The positive outcomes of this study may be attributed to the dosing of the probiotics administered, which is 80 times higher than the amount Lactobacillus spp. Recommended to restore and maintain a normal intravaginal microbiota [32].
In another study by Ozkinay et al. [12], women with vaginal infections were recruited. A vaginal tablet containing at least 107 CFU of L. acidophilus, 0.03 mg of estriol and 600 mg of lactose was administered daily for a period of 6 days in pre-menopausal women and for 12 days in post-menopausal women. The study reported that exogenously administered live probiotics in combination with low-dose estriol significantly improved the restoration of the vaginal microbiota. Ehrstrom et al. [14] also demonstrated for the first time that a short period of probiotics administration (5 days) can lead to a colonization of exogenous lactobacilli in the vaginal canal for up to 6 months. The restitution of the exogenous bacteria leads to competitive exclusion, in which serious prohibition of adhesion or displacement of pathogens takes place. The probiotics and pathogenic microbes might compete for nutrients and adhesion sites in the vaginal canal. Consequently, probiotics inhibit the development and colonization of pathogenic bacteria, viruses, or fungi by occupying these sites and utilizing the resources that are available, thus preserving a balanced microbiome and creating an inhospitable environment for the growth of pathogens [33].
Other studies confirm the influence of Lactobacilli spp. as an adjuvant to conventional treatment with antibiotics [10][11]. In a study by Bradshaw et al. [10], symptomatic BV-positive women were recruited and vaginal probiotic cream containing L. acidophilus KS400 was administered to evaluate the efficacy of probiotics as adjuvants following oral metronidazole treatment on the recurrence rate of BV. It was reported that 7 days of oral metronidazole treatment in combination with a 2% vaginal clindamycin cream, or a 12-day treatment with vaginal probiotic cream did not achieve higher cure rates for BV compared with oral metronidazole monotherapy over a six-month follow-up period. However, improved restoration of normal vaginal microbiota was demonstrated in another study by Mastromarino et al. [11], which evaluated the administration of antimicrobial metronidazole therapy for BV with oral probiotic capsules containing L. rhamnosus GR-1 and L. reuteri RC-14 for 30 days. It was found that 88% were cured in the antibiotic/probiotic group compared to 40% in the antibiotic/placebo group.
Restoration of balanced vaginal microbiota was also reported by Vujic et al. [13] after the administration of probiotics in the form of oral capsules containing more than 109 CFU Lactobacillus rhamnosus GR-1 and L-reuteri RC-14 in 243 subjects (61.52%) in the probiotic group. The restoration of a balanced vaginal microbiota was highly coincident with the presence of lactobacilli in the vaginal swabs, as high counts (>105 CFU/mL) were found in 81.5% of subjects who received probiotics. These studies have thus shown that the overwhelming probiotic load can strongly influence the vaginal microbiota, and thus, repopulate the vagina with appropriate concentration of Lactobacilli sp., inhibit the growth of pathogenic microbes and prevent G. vaginalis and other anaerobes from adhering to the vaginal canal [9]. Probiotic strains, especially Lactobacillus spp., have antimicrobial properties due to their ability to produce organic acids. The repression of pathogenic growth happens because of the ability of probiotics to emit lactic acid and acetic acid. These acids lower the pH of the microbial environment and it becomes overly acidic, thus barring any pathogenic microbes that are incapable of withstanding an acidic environment [34].
2.2. Effects of Probiotics on PCOS
Polycystic ovarian syndrome (PCOS) is a complex gynaecological endocrine disorder with significant and diverse clinical implications, affecting reproductive functions (infertility, hyperandrogenism, hirsutism), metabolic functions (insulin resistance, type 2 diabetes mellitus, impaired glucose tolerance, adverse cardiovascular risk profiles) and psychological features (depression, increased anxiety, deterioration in quality of life) [35]. Its prevalence varies from country to country and depends on clinical and biochemical features that differ by races and age group [36][37].
Currently, the treatment for PCOS typically involves a multidimensional approach targeting different aspects of the disorders. There are various interventions that can take place to manage symptoms and improve the quality of life, such as dietary and lifestyle modifications, medications usage, and the use of in vitro fertilization (IVF), or surgical options to improve reproductive outcomes [38][39]. Although these interventions aim to address specific aspects of the condition, such as insulin resistance, hormonal imbalances, and infertility, they may not eliminate the underlying hormonal and metabolic abnormalities associated with PCOS. Most of these studies demonstrated positive effects on the health of women with PCOS, suggesting that probiotics supplementation may be used as an alternative or complementary treatment for PCOS. The studies suggest that probiotic supplementation may influence weight loss, biomarkers of insulin resistance, and lipid profiles in PCOS patients. These beneficial effects could be mediated by host metabolism, modulation of immunological responses and reduction in systemic inflammation [36].
Obesity is one of the factors that may increase the risk of PCOS by promoting hyperandrogenism, hirsutism, and infertility [40]. About 40–80% of PCOS patients are overweight or obese, which increases the likelihood these women will suffer from reproductive dysfunction (irregular menstruation and infertility issues) and various psychological disorders (anxiety, sadness and bipolar disorder) [40][41]. However, some of these features of PCOS are often reversible through lifestyle modifications, such as implementing an exercise routine and nutritional supplementation, as demonstrated in studies by Ahmadi et al. and Ghanei et al. [15][20].
In a clinical trial conducted by Ahmadi et al. [15], a probiotic capsule, consisting of three viable and freeze-dried strains, Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g), and Bifidobacterium bifidum (2 × 109 CFU/g), was administered daily to 60 PCOS patients for 12 weeks. The study demonstrated a significant reduction in the weight and BMI of the PCOS patients after 12 weeks of supplementation with probiotics compared to the placebo. The weight loss was associated with a significant decrease in FPG, serum insulin concentrations, HOMA-IR, HOMA-B, serum triglycerides and VLDL–cholesterol. This could be due to the hypocholesterolaemia effect of the probiotics [36].
Another study by Ghanei et al. [20] also supports this finding, showing that 12 weeks of treatment with Cypretrone acetate and 1 × 109 CFU/g of L. acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, and Lactobacillus gasseri resulted in significant weight loss and reduction in BMI compared to the placebo group. These results are consistent with a previous study by Sanchez et al. [42], which indicated that treatment with Lactobacillus rhamnosus in conjunction with a controlled energy intake is a helpful modulator for weight management associated with obesity, in both men and women, by lowering plasma leptin concentrations directly or through changes in microbiota composition or function. However, in five clinical trials involving probiotic supplementation and PCOS patients, no change in body weight and BMI was recorded [16][17][18][19][21].
As obesity often exacerbates PCOS symptoms, the use of probiotics and a comprehensive weight-loss program have been linked with improved body composition and modest weight loss. Studies suggest that PCOS patients are more prone to metabolic disturbances, such as hyperandrogenism, increased inflammatory factors, insulin resistance and oxidative stress parameters [35]. These conditions eventually lead to a change in the overall composition of the gut microbiota, specifically causing an alteration in Alpha (α) and Beta (β) diversity. α diversity can be used to estimate the abundance of species in a microbiome, while β diversity refers to the variety of the ecological environment [43]. The decrease in α diversity and changes in β diversity in patients with PCOS may lead to changes in intestinal functions, activates the immune system, which interferes with the insulin receptor function, and increases androgen production in the ovaries, which in turn prevents normal follicle development [35]. Thus, as a result, the dysbiosis of gut microbiota occurs. Therefore, it has been suggested that the intake of probiotics may be useful in restoring the normal homeostasis of the gut microbiota.
In a study by Nasri et al. [17], a significant decrease in serum SHBG, insulin levels, and HOMA-IR was found after 12 weeks of supplementation with Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum (2 ×109 CFU/g each) capsules. These results are consistent with the findings of Shoaie et al. [21], who indicated that the fasting glucose and insulin resistance significantly improved in PCOS patients with metabolic syndrome after 12 weeks of probiotic supplementation. This is consistent with the findings from studies linking short-chain fatty acids, bile acid metabolism and endotoxemia, a persistent inflammatory response, with the occurrence of insulin resistance [44]. As significant imbalance in the intestinal flora has also been observed in PCOS patients [45][46], it can be speculated that the gut microbiota may be involved in the pathogenesis of PCOS by influencing insulin resistance and systemic low-grade inflammation, altering the sex hormones and other pathological mechanisms.
Rashad et al. [19] also conducted a study wherein patients with PCOS were given 10 billion CFU probiotic capsules containing Lactobacillus delbruekii and Lactobacillus fermentum twice daily for 12 weeks. It was reported that the intake of probiotics in PCOS patients led to a significant improvement in the glucose parameters, such as fasting blood glucose, compared to baseline. There was also a significant positive impact on the lipid profile (p < 0.05) with a significant decrease in detected inflammatory biomarkers. These changes in the lipid metabolism and insulin resistance might be attributed to the maintenance of homeostasis in the internal microbiota as probiotics restabilize dysbiosis [47]. These findings correlated with the study by Jamilian et al. [18], which also reported significant improvement in hirsutism, total testosterone, and sex hormone-binding globulin (SHBG) levels after 12 weeks of selenium and probiotics administration. Since most PCOS patients suffer from metabolic abnormalities, treatments with probiotics may provide significant relief for these patients. As demonstrated by the studies above, probiotics can decrease the production of androgens by increasing the levels of SHBG that bind and regulate free testosterone, subsequently improving hirsutism symptoms [48].
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Hotel, A.C.P.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10.
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365s–373s.
- Fuller, R.; Tannock, G.W. Probiotics a Critical Review; Horizon Scientific: Wymondham, UK, 1999; pp. 15–22.
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651.
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021.
- Homayouni, A.; Bastani, P.; Ziyadi, S.; Mohammad-Alizadeh-Charandabi, S.; Ghalibaf, M.; Mortazavian, A.M.; Mehrabany, E.V. Effects of Probiotics on the Recurrence of Bacterial Vaginosis: A Review. J. Low. Genit. Tract Dis. 2014, 18, 79–86.
- Buggio, L.; Somigliana, E.; Borghi, A.; Vercellini, P. Probiotics and vaginal microecology: Fact or fancy? BMC Womens Health 2019, 19, 25.
- Ya, W.; Reifer, C.; Miller, L.E. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: A double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010, 203, 120.e1–120.e6.
- Bradshaw, C.S.; Pirotta, M.; De Guingand, D.; Hocking, J.S.; Morton, A.N.; Garland, S.M.; Fehler, G.; Morrow, A.; Walker, S.; Vodstrcil, L.A.; et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: Randomised placebo-controlled double-blind trial. PLoS ONE 2012, 7, e34540.
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol. 2013, 36, 229–238.
- Ozkinay, E.; Terek, M.C.; Yayci, M.; Kaiser, R.; Grob, P.; Tuncay, G. The effectiveness of live lactobacilli in combination with low dose oestriol (Gynoflor) to restore the vaginal flora after treatment of vaginal infections. BJOG 2005, 112, 234–240.
- Vujic, G.; Jajac Knez, A.; Despot Stefanovic, V.; Kuzmic Vrbanovic, V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 75–79.
- Ehrstrom, S.; Daroczy, K.; Rylander, E.; Samuelsson, C.; Johannesson, U.; Anzen, B.; Pahlson, C. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 2010, 12, 691–699.
- Ahmadi, S.; Jamilian, M.; Karamali, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Hum. Fertil. 2017, 20, 254–261.
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2022, 14, 1–14.
- Nasri, K.; Jamilian, M.; Rahmani, E.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of synbiotic supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. BMC Endocr. Disord. 2018, 18, 21.
- Jamilian, M.; Mansury, S.; Bahmani, F.; Heidar, Z.; Amirani, E.; Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J. Ovarian Res. 2018, 11, 80.
- Rashad, N.M.; El-Shal, A.S.; Amin, A.I.; Soliman, M.H. Effects of probiotics supplementation on macrophage migration inhibitory factor and clinical laboratory feature of polycystic ovary syndrome. J. Funct. Foods 2017, 36, 317–324.
- Ghanei, N.; Rezaei, N.; Amiri, G.A.; Zayeri, F.; Makki, G.; Nasseri, E. The probiotic supplementation reduced inflammation in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J. Funct. Foods 2018, 42, 306–311.
- Shoaei, T.; Heidari-Beni, M.; Tehrani, H.G.; Feizi, A.; Esmaillzadeh, A.; Askari, G. Effects of Probiotic Supplementation on Pancreatic beta-cell Function and C-reactive Protein in Women with Polycystic Ovary Syndrome: A Randomized Double-blind Placebo-controlled Clinical Trial. Int. J. Prev. Med. 2015, 6, 27.
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183.
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210.
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881.
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902.
- Kim, J.M.; Park, Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal Med. 2017, 23, 139–145.
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972.
- Maretti, C.; Cavallini, G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: A pilot study. Andrology 2017, 5, 439–444.
- Reid, J.N.; Bisanz, J.E.; Monachese, M.; Burton, J.P.; Reid, G. The rationale for probiotics improving reproductive health and pregnancy outcome. Am. J. Reprod. Immunol. 2013, 69, 558–566.
- Chen, R.; Li, R.; Qing, W.; Zhang, Y.; Zhou, Z.; Hou, Y.; Shi, Y.; Zhou, H.; Chen, M. Probiotics are a good choice for the treatment of bacterial vaginosis: A meta-analysis of randomized controlled trial. Reprod. Health 2022, 19, 137.
- Wang, Z.; He, Y.; Zheng, Y. Probiotics for the Treatment of Bacterial Vaginosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3859.
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38.
- Machado, A.; Jefferson, K.K.; Cerca, N. Interactions between Lactobacillus crispatus and bacterial vaginosis (BV)-associated bacterial species in initial attachment and biofilm formation. Int. J. Mol. Sci. 2013, 14, 12004–12012.
- Iqbal, Z.; Ahmed, S.; Tabassum, N.; Bhattacharya, R.; Bose, D. Role of probiotics in prevention and treatment of enteric infections: A comprehensive review. 3 Biotech 2021, 11, 242.
- Teede, H.; Deeks, A.; Moran, L. Review Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41–50.
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271.
- Yasmin, A.; Roychoudhury, S.; Paul Choudhury, A.; Ahmed, A.B.F.; Dutta, S.; Mottola, F.; Verma, V.; Kalita, J.C.; Kumar, D.; Sengupta, P.; et al. Polycystic Ovary Syndrome: An Updated Overview Foregrounding Impacts of Ethnicities and Geographic Variations. Life 2022, 12, 1974.
- Committee on Practice Bulletins—Gynecology. Polycystic ovary syndrome. Obstet. Gynecol. 2018, 131, e157–e171.
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine, S. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592.
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042.
- Barber, T.M. Why are women with polycystic ovary syndrome obese? Br. Med. Bull. 2022, 143, 4–15.
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519.
- He, Y.; Wang, Q.; Li, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020, 11, 5192–5204.
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73.
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology Bethesda 2016, 31, 283–293.
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381.
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521.
- Lolou, V. The Role of Probiotics and Synbiotics on Hirsutism. Fermentation 2021, 7, 10.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
934
Revisions:
2 times
(View History)
Update Date:
30 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




