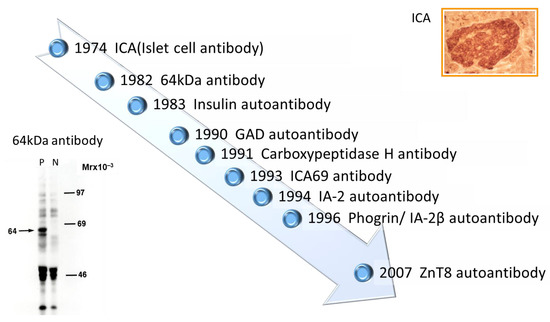
2. History of Anti-Islet Autoantibody Discovery
Discovery of islet cell antibodies (ICA) as the first anti-islet autoantibodies in T1D was made by Bottazzo and coworkers in 1974
[5]. ICA detection involved using indirect immunofluorescence on the frozen pancreatic tissue sections of human blood group O, which may recognize various autoantigens. In 1982, Baekkeskov and coworkers discovered autoantibodies against an islet protein with a molecular weight of 64,000 (64 kDa antibody) using the immunoprecipitation method and
35S-methionine-labeled human islet cells
[6]. Moreover, in 1983, Palmer and coworkers reported insulin autoantibody (IAA) in insulin naïve new-onset patients with T1D, measured by polyethylene glycol competitive assay using
125I-Tyr A14 human monoiodinated insulin
[7].
To overcome the limitation of ICA assay, such as being time-consuming, requiring human pancreatic tissue, and yielding difficulty in obtaining quantitative results, extensive efforts have been made to identify target antigens against ICA using advanced molecular biological techniques such as molecular cloning, gel electrophoresis, polymerase chain reaction, and DNA microarray analysis. To date, more than 10 target antigens have been discovered (
Table 1). After identifying the 64kDa islet protein as glutamic acid decarboxylase (GAD) in 1990
[8], several autoantibodies (
Figure 1) were discovered. Currently, in addition to IAA and GAD autoantibodies (GADA), tyrosine phosphatase-like protein IA-2 autoantibodies (IA-2A)
[9] and zinc transporter 8 autoantibodies (ZnT8A)
[10] are employed for the diagnosis, pathological analysis, and prediction of T1D. Additionally, detection methods have evolved from immunohistochemical staining used in ICA to the radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and electrochemiluminescence (ECL) assay using recombinant autoantigens produced via prokaryotic and eukaryotic expression systems or in vitro transcription/translation system.
Figure 1. Chronology of anti-islet autoantibody discovery. Anti-islet autoantibodies used for prediction and diagnoses of T1D are IAA, GADA, IA-2A, and ZnT8A.
Table 1. Localization and function of autoantigens against anti-islet autoantibodies.
| Name of Antigen |
Localization |
Function |
Reference |
| Insulin |
Insulin secretory granules |
Regulate glucose levels in the blood and induce glucose storage in the liver, muscles, and adipose tissue |
[7] |
| GAD65 |
Synaptic-like vesicles in the cytoplasm of β-cells |
Rate-limiting enzyme engaged in the synthesis of the neurotransmitter γ-aminobutyric acid from L-glutamate |
[8] |
| GAD67 |
Cytosol of β-cells |
Rate-limiting enzyme engaged in the synthesis of the neurotransmitter γ-aminobutyric acid from L-glutamate |
[11] |
| IA-2 |
Insulin secretory granule membrane |
Regulate insulin secretory granule content and β-cell growth |
[9][12] |
| Phogrin/IA-2β |
Insulin secretory granule membrane |
Regulate insulin secretory granule content and β-cell growth |
[13][14] |
| Carboxypeptidase H |
Insulin secretory granules and granule membrane |
Convert proinsulin into insulin and C-peptide by catalyzing the release of C-terminal arginine or lysine residues from polypeptides |
[15] |
| ICA69 |
Insulin secretory granule membrane |
Dense-core vesicles signaling and maturation |
[16] |
| ZnT8 |
Insulin secretory granule membrane |
Transport zinc ion from the cytosol into the insulin secretory granules |
[17][18] |
| GM2-1 ganglioside |
Secretory granules in β-cells and non-β-cells |
unknown |
[19] |
| Heat shock protein 60 |
Insulin secretory granules |
Assist correct folding of partially folded polypeptides and presentation of antigen to MHC molecules |
[20] |
| GLUT2 |
β-cell surface membrane |
Uptake glucose from the blood into β-cells |
[21] |
| Tetraspanin-7 |
Insulin secretory granule membrane |
Regulate Ca2+-dependent insulin exocytosis |
[22] |
| ICA12/SOX13 |
Cytoplasm and nucleus in β-cells and non-β-cells |
Transcription factor (Function in the islets is unknown) |
[23] |
3. Pathophysiology of the Generation of Anti-Islet Autoantibodies
Although the role of B cells in the pathogenesis of T1D has been extensively studied in non-obese diabetic (NOD) mice, an animal model of human T1D, the mechanism of anti-islet autoantibody generation in humans remains largely unexplored and is currently unknown. However, it is assumed that genetic background, such as MHC class II gene, protein tyrosine phosphatase type 22 gene, and interleukin-2 receptor α gene, and environmental factors are associated with the anti-islet autoantibody generation
[24]. In the NOD mice, it has been reported that the escape of islet-specific T cells into the periphery reduced regulatory T cell number and function, and the increased production of autoreactive B cells are thought to play key roles in anti-islet autoantibody generation
[24][25]. Pinto and coworkers reported that increased numbers of thymic B cells and the formation of thymic germinal centers lead to a substantial increase in intrathymic autoantibody levels, resulting in the loss of certain medullary thymic epithelial cells, a decreased negative selection of autoreactive T cells, and an enhanced survival of insulin-reactive thymocytes
[26].
4. Localization and Function of Autoantigens against Anti-Islet Autoantibodies
- (1)
-
Insulin
Insulin is a peptide hormone converted from proinsulin in pancreatic β-cells. Proinsulin consists of an A-chain (21 amino acids), a B-chain (30 amino acids), and a C-peptide (31 amino acids), and mature insulin is generated after C-peptide is excised by a series of proteolytic cleavages. The function of insulin is to regulate glucose levels in the blood and induce glucose storage in the liver, muscles, and adipose tissue.
- (2)
-
GAD 65 and GAD67
GAD is a rate-limiting enzyme that catalyzes a decarboxylation reaction to produce the neurotransmitter γ-aminobutyric acid from L-glutamate. There are two isoforms of GAD, GAD65 (585 amino acids), and GAD67 (594 amino acids), both of which are abundant in the central nervous system and pancreatic islets. In pancreatic β-cells, GAD65 and GAD67 are localized in the synaptic-like vesicles and cytosol, respectively.
- (3)
-
IA-2 and IA-2β/phogrin
IA-2 (also known as ICA512) is a transmembrane glycoprotein of the protein tyrosine phosphatase (PTP) family that colocalizes with IA-2β/phogrin and is expressed in insulin-secretory granule membranes in pancreatic β-cells
[9][13]. IA-2 consists of 979 amino acids and IA-2β/phogrin consists of 1015 amino acids. Both polypeptides regulate insulin secretory granule content and pancreatic β-cell growth.
- (4)
-
ZnT8
ZnT8, a member of the zinc transporter family, is specifically localized in the insulin-secretory granule membrane of pancreatic β-cells and consists of 369 amino acids. ZnT8 is responsible for transporting zinc influx from the cytoplasm into insulin granules, facilitating insulin hexamer formation
[17][18]. Zinc ions transported into the insulin-secreting granule cavity by ZnT8 are used to stabilize insulin hexamers, and zinc ions ejected outside the cells during insulin secretion have an autocrine effect on β-cells, or alternatively, exert a paracrine action on α-cells.
- (5)
-
Carboxypeptidase H
Carboxypeptidase H is an enzyme which converts proinsulin into insulin and C-peptide by catalyzing the release of C-terminal arginine or lysine residues from polypeptides. This enzyme is localized in the insulin secretory granules and granule membrane of pancreatic β-cells.
- (6)
-
ICA69
Islet-cell autoantigen 69 (ICA69) is localized in the insulin secretory granule membrane and consists of 483 amino acids. This protein is involved in the signaling and maturation of dene-core vesicles.
- (7)
-
GM2-1 ganglioside
GM2-1 ganglioside (N-acetyl neuraminic acid-galactose-galactosamine-galactosamine-glucose-ceramide) is a pancreatic monosialoganglioside migrating between GM2 and GM1 standards which is localized in the secretory granules in β-cells and non-β-cells. However, the function of GM2-1 ganglioside is still unknown.
- (8)
-
Heat shock protein 60
Heat shock proteins were originally described as “cellular stress responders” for their role as chaperones. Heat shock protein 60 (HSP60) consists of 569 amino acids and is localized in the insulin secretory granules. This protein assists in the correct folding of partially folded polypeptides and the presentation of antigen to MHC molecules.
- (9)
-
GLUT2
Glucose transporter-2 (GLUT2) is a subclass of GLUTs and consists of 524 amino acids. GLUT2 is expressed mainly in pancreatic β-cells of the pancreas, liver, and kidney. This transporter is localized on the β-cell surface membrane and is responsible for the transport of glucose from blood into β-cells.
- (10)
-
Tetraspanin-7
Tetraspanin-7 is expressed in the insulin-containing granule membranes of pancreatic β-cells and glucagon-producing α-cells. This protein consists of 249 amino acids and is involved in the regulation of Ca2+-dependent insulin exocytosis.
- (11)
-
ICA12/SOX13
ICA12/SOX13 belongs to the class D subgroup of SOX transcription factors and is localized in the cytoplasm and nucleus in β-cells and non-β-cells. ICA12/SOX13 consists of 604 amino acids and contains a leucine zipper domain. The function of this transcription factor in the islets is still unknown.
5. Significance of Anti-Islet Autoantibodies in the Pathophysiology of T1D
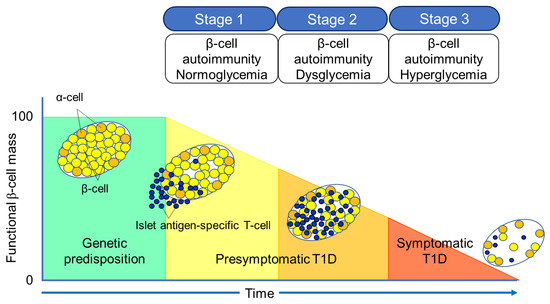
The Juvenile Diabetes Research Foundation (JDRF), American Diabetes Association (ADA), and Endocrine Society recommend classifying the natural history of T1D into three stages
[27] (
Figure 2). In Stage 1, autoimmunity to pancreatic islet cells is induced by cytotoxic T cells, and the destruction of pancreatic β-cells (insulitis) begins, even though blood glucose levels remain within the normal range. Two or more IAA, GADA, IA-2A, and ZnT8A, emerge in this stage, with the five-year and ten-year risks of T1D development being approximately 44% and 70%, respectively, while the lifetime risk approaches 100%
[28]. However, it is currently understood that anti-islet autoantibodies do not directly cause pancreatic β-cell destruction; they arise as the result of β-cell destruction mediated by T-cells. In Stage 2, similar to Stage 1, individuals test positive for two or more anti-islet autoantibodies, and β-cell destruction progresses, leading to glucose intolerance or dysglycemia. The five-year risk of T1D development at this stage is approximately 75%, and the lifetime risk approaches 100%
[29]. Stage 3 is marked by the manifestations of typical clinical symptoms and signs of T1D, including polyuria, polydipsia, weight loss, general fatigue, diabetic ketoacidosis, and others. In this stage, the amount of residual pancreatic β-cells decreases to 20–30% of normal levels. Since anti-islet autoantibodies appear in the peripheral blood during Stage 1 and Stage 2, they are used as a tool for predicting the onset of T1D. The number of positive autoantibodies is more important for prediction than the specific positive autoantibodies
[30].
Figure 2. Three stages of natural history of T1D.
Additionally, a study involving first-degree relatives of patients with T1D reported that IAA or GADA appeared first, followed by IA-2A and ZnT8A directly before the onset of diabetes
[31]. We observed a similar sequential emergence of anti-islet autoantibodies in patients with T1D who developed the condition during interferon therapy
[32]. Based on this evidence, IA-2A and ZnT8A are considered surrogate markers for pancreatic β-cell destruction. This is supported by a report demonstrating the epitope spreading of IA-2A during the preclinical stage of T1D
[33].
Conversely, as shown in
Table 2, GADA is detected not only in patients with acute-onset T1D and SPIDDM, but also in some patients with fulminant T1D and non-diabetic patients with polyglandular autoimmune syndrome, autoimmune thyroid disease (AITD), and stiff-person syndrome. Therefore, unlike IA-2A and ZnT8A, it is presumed that GADA may not be a specific marker for pancreatic β-cell destruction. In our case, where GADA became re-elevated more than 10 years after the onset of T1D, anti-thyroid autoantibodies turned positive directly before the re-elevation of GADA
[34]. Therefore, it is important to note that GADA may be associated with thyroid autoimmunity rather than insulitis in some instances.
Table 2. Disease specificity of GAD autoantibodies.
| Subject |
Prevalence |
| Healthy control |
<1% |
| Acute-onset type 1 diabetes (at onset) |
60–80% |
| Fulminant type 1 diabetes |
5–9% |
| LADA (SPIDDM) |
100% |
| Type 2 diabetes (diet/OHA) |
4–5% |
| Polyglandular autoimmune syndrome, type 1 |
30–40% |
| Polyglandular autoimmune syndrome, type 2 |
30–50% |
| Autoimmune thyroid disease |
6–8% |
| Stiff-person syndrome |
60–70% |
6. Role of Anti-Islet Autoantibodies in the Diagnosis of T1D
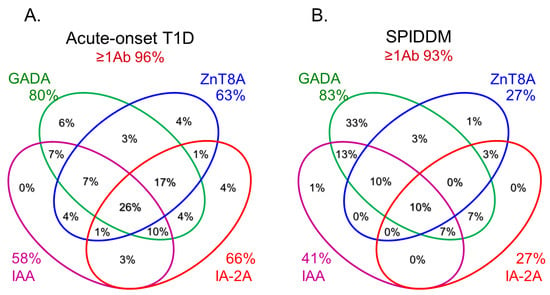
In this section, we discuss the key considerations when diagnosing T1D using anti-islet autoantibodies. First, because distinguishing IAA from insulin antibodies produced by exogenous insulin injection is challenging, we considered patients positive for IAA if antibodies to insulin were present in insulin-naïve patients or those within 2 weeks of initiating insulin therapy. Our study using sera obtained within 2 weeks after onset revealed that the prevalence of IAA, GADA, IA-2A, and ZnT8A in patients with acute-onset T1D was 58, 80, 66, and 63%, respectively (
Figure 3A). Similarly, the prevalence of these autoantibodies in SPIDDM patients was 41, 83, 27, and 27%, respectively (
Figure 3B). Thus, a combinatorial analysis of the four autoantibodies indicated that 96% of acute-onset T1D and 93% of SPIDDM were immune-mediated T1D, while approximately 5% were classified as idiopathic T1D
[35]. These data are consistent with previous reports from studies conducted in countries with Caucasoid populations
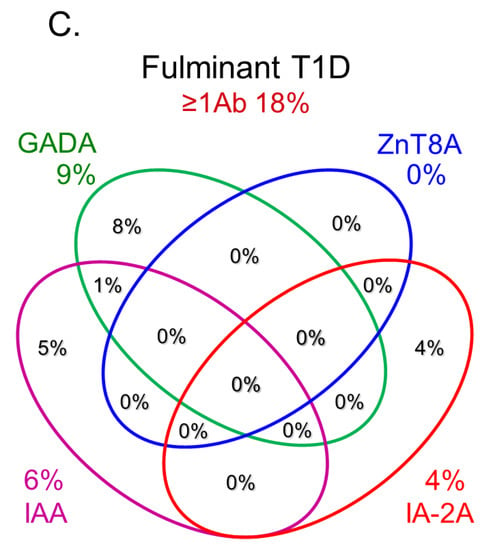
[10][36][37][38]. In contrast, the majority of patients with fulminant T1D were negative for anti-islet autoantibodies (
Figure 3C) and are classified into idiopathic T1D, suggesting that the mechanism of β-cell destruction in fulminant T1D may differ from acute-onset T1D and SPIDDM.
Figure 3. Combinatorial analysis of anti-islet autoantibodies in patients with acute-onset T1D (
A), SPIDDM (
B), and fulminant T1D (
C) Adapted with permission from Ref.
[35].
In Japan, medical insurance guidelines instruct physicians to measure GADA first when T1D is suspected. However, about 10% of non-fulminant T1D patients are positive for other anti-islet autoantibodies without GADA. Therefore, measuring multiple autoantibodies is crucial for the accurate diagnosis of immune-mediated T1D.