Anti-islet autoantibodies serve as key markers in immune-mediated type 1 diabetes (T1D) and slowly progressive T1D (SPIDDM), also known as latent autoimmune diabetes in adults (LADA). Autoantibodies to insulin (IAA), glutamic acid decarboxylase (GADA), tyrosine phosphatase-like protein IA-2 (IA-2A), and zinc transporter 8 (ZnT8A) are currently employed in the diagnosis, pathological analysis, and prediction of T1D. GADA can also be detected in non-diabetic patients with autoimmune diseases other than T1D and may not necessarily reflect insulitis. Conversely, IA-2A and ZnT8A serve as surrogate markers of pancreatic β-cell destruction. A combinatorial analysis of these four anti-islet autoantibodies demonstrated that 93–96% of acute-onset T1D and SPIDDM cases were diagnosed as immune-mediated T1D, while the majority of fulminant T1D cases were autoantibody-negative. Evaluating the epitopes and immunoglobulin subclasses of anti-islet autoantibodies help distinguish between diabetes-associated and non-diabetes-associated autoantibodies and is valuable for predicting future insulin deficiency in SPIDDM (LADA) patients.
- enzyme-linked immunosorbent assay
- epitope
- glutamic acid decarboxylase
- Latent-Autoimmune Diabetes in Adults (LADA)
- Slowly-progressive type 1 diabetes (SPIDDM)
1. Introduction
2. History of Anti-Islet Autoantibody Discovery
Discovery of islet cell antibodies (ICA) as the first anti-islet autoantibodies in T1D was made by Bottazzo and coworkers in 1974 [5]. ICA detection involved using indirect immunofluorescence on the frozen pancreatic tissue sections of human blood group O, which may recognize various autoantigens. In 1982, Baekkeskov and coworkers discovered autoantibodies against an islet protein with a molecular weight of 64,000 (64 kDa antibody) using the immunoprecipitation method and 35S-methionine-labeled human islet cells [6]. Moreover, in 1983, Palmer and coworkers reported insulin autoantibody (IAA) in insulin naïve new-onset patients with T1D, measured by polyethylene glycol competitive assay using 125I-Tyr A14 human monoiodinated insulin [7]. To overcome the limitation of ICA assay, such as being time-consuming, requiring human pancreatic tissue, and yielding difficulty in obtaining quantitative results, extensive efforts have been made to identify target antigens against ICA using advanced molecular biological techniques such as molecular cloning, gel electrophoresis, polymerase chain reaction, and DNA microarray analysis. To date, more than 10 target antigens have been discovered (Table 1). After identifying the 64kDa islet protein as glutamic acid decarboxylase (GAD) in 1990 [8], several autoantibodies (Figure 1) were discovered. Currently, in addition to IAA and GAD autoantibodies (GADA), tyrosine phosphatase-like protein IA-2 autoantibodies (IA-2A) [9] and zinc transporter 8 autoantibodies (ZnT8A) [10] are employed for the diagnosis, pathological analysis, and prediction of T1D. Additionally, detection methods have evolved from immunohistochemical staining used in ICA to the radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and electrochemiluminescence (ECL) assay using recombinant autoantigens produced via prokaryotic and eukaryotic expression systems or in vitro transcription/translation system.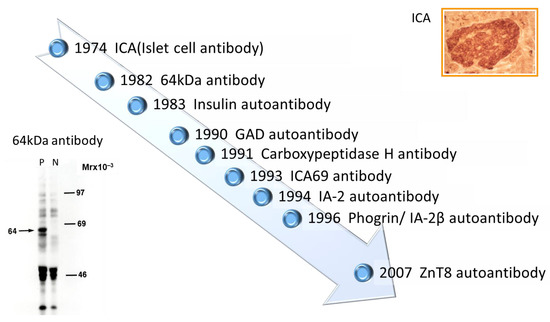
| Name of Antigen | Localization | Function | Reference |
|---|---|---|---|
| Insulin | Insulin secretory granules | Regulate glucose levels in the blood and induce glucose storage in the liver, muscles, and adipose tissue | [7] |
| GAD65 | Synaptic-like vesicles in the cytoplasm of β-cells | Rate-limiting enzyme engaged in the synthesis of the neurotransmitter γ-aminobutyric acid from L-glutamate | [8] |
| GAD67 | Cytosol of β-cells | Rate-limiting enzyme engaged in the synthesis of the neurotransmitter γ-aminobutyric acid from L-glutamate | [11] |
| IA-2 | Insulin secretory granule membrane | Regulate insulin secretory granule content and β-cell growth | [9][12][9,12] |
| Phogrin/IA-2β | Insulin secretory granule membrane | Regulate insulin secretory granule content and β-cell growth | [13][14][13,14] |
| Carboxypeptidase H | Insulin secretory granules and granule membrane | Convert proinsulin into insulin and C-peptide by catalyzing the release of C-terminal arginine or lysine residues from polypeptides | [15] |
| ICA69 | Insulin secretory granule membrane | Dense-core vesicles signaling and maturation | [16] |
| ZnT8 | Insulin secretory granule membrane | Transport zinc ion from the cytosol into the insulin secretory granules | [17][18][17,18] |
| GM2-1 ganglioside | Secretory granules in β-cells and non-β-cells | unknown | [19] |
| Heat shock protein 60 | Insulin secretory granules | Assist correct folding of partially folded polypeptides and presentation of antigen to MHC molecules | [20] |
| GLUT2 | β-cell surface membrane | Uptake glucose from the blood into β-cells | [21] |
| Tetraspanin-7 | Insulin secretory granule membrane | Regulate Ca2+-dependent insulin exocytosis | [22] |
| ICA12/SOX13 | Cytoplasm and nucleus in β-cells and non-β-cells | Transcription factor (Function in the islets is unknown) | [23] |
3. Pathophysiology of the Generation of Anti-Islet Autoantibodies
Although the role of B cells in the pathogenesis of T1D has been extensively studied in non-obese diabetic (NOD) mice, an animal model of human T1D, the mechanism of anti-islet autoantibody generation in humans remains largely unexplored and is currently unknown. However, it is assumed that genetic background, such as MHC class II gene, protein tyrosine phosphatase type 22 gene, and interleukin-2 receptor α gene, and environmental factors are associated with the anti-islet autoantibody generation [24]. In the NOD mice, it has been reported that the escape of islet-specific T cells into the periphery reduced regulatory T cell number and function, and the increased production of autoreactive B cells are thought to play key roles in anti-islet autoantibody generation [24][25][24,25]. Pinto and coworkers reported that increased numbers of thymic B cells and the formation of thymic germinal centers lead to a substantial increase in intrathymic autoantibody levels, resulting in the loss of certain medullary thymic epithelial cells, a decreased negative selection of autoreactive T cells, and an enhanced survival of insulin-reactive thymocytes [26].4. Localization and Function of Autoantigens against Anti-Islet Autoantibodies
- (1)
-
Insulin
- (2)
-
GAD 65 and GAD67
- (3)
-
IA-2 and IA-2β/phogrin
- (4)
-
ZnT8
- (5)
-
Carboxypeptidase H
- (6)
-
ICA69
- (7)
-
GM2-1 ganglioside
- (8)
-
Heat shock protein 60
- (9)
-
GLUT2
- (10)
-
Tetraspanin-7
- (11)
-
ICA12/SOX13
5. Significance of Anti-Islet Autoantibodies in the Pathophysiology of T1D
The Juvenile Diabetes Research Foundation (JDRF), American Diabetes Association (ADA), and Endocrine Society recommend classifying the natural history of T1D into three stages [27] (Figure 2). In Stage 1, autoimmunity to pancreatic islet cells is induced by cytotoxic T cells, and the destruction of pancreatic β-cells (insulitis) begins, even though blood glucose levels remain within the normal range. Two or more IAA, GADA, IA-2A, and ZnT8A, emerge in this stage, with the five-year and ten-year risks of T1D development being approximately 44% and 70%, respectively, while the lifetime risk approaches 100% [28]. However, it is currently understood that anti-islet autoantibodies do not directly cause pancreatic β-cell destruction; they arise as the result of β-cell destruction mediated by T-cells. In Stage 2, similar to Stage 1, individuals test positive for two or more anti-islet autoantibodies, and β-cell destruction progresses, leading to glucose intolerance or dysglycemia. The five-year risk of T1D development at this stage is approximately 75%, and the lifetime risk approaches 100% [29]. Stage 3 is marked by the manifestations of typical clinical symptoms and signs of T1D, including polyuria, polydipsia, weight loss, general fatigue, diabetic ketoacidosis, and others. In this stage, the amount of residual pancreatic β-cells decreases to 20–30% of normal levels. Since anti-islet autoantibodies appear in the peripheral blood during Stage 1 and Stage 2, they are used as a tool for predicting the onset of T1D. The number of positive autoantibodies is more important for prediction than the specific positive autoantibodies [30].Additionally, a study involving first-degree relatives of patients with T1D reported that IAA or GADA appeared first, followed by IA-2A and ZnT8A directly before the onset of diabetes [31]. We observed a similar sequential emergence of anti-islet autoantibodies in patients with T1D who developed the condition during interferon therapy [32]. Based on this evidence, IA-2A and ZnT8A are considered surrogate markers for pancreatic β-cell destruction. This is supported by a report demonstrating the epitope spreading of IA-2A during the preclinical stage of T1D [33]. Conversely, as shown in Table 2, GADA is detected not only in patients with acute-onset T1D and SPIDDM, but also in some patients with fulminant T1D and non-diabetic patients with polyglandular autoimmune syndrome, autoimmune thyroid disease (AITD), and stiff-person syndrome. Therefore, unlike IA-2A and ZnT8A, it is presumed that GADA may not be a specific marker for pancreatic β-cell destruction. In our case, where GADA became re-elevated more than 10 years after the onset of T1D, anti-thyroid autoantibodies turned positive directly before the re-elevation of GADA [34]. Therefore, it is important to note that GADA may be associated with thyroid autoimmunity rather than insulitis in some instances.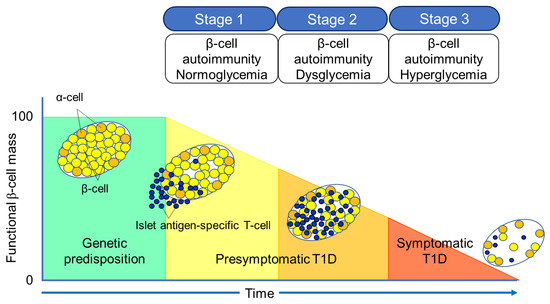 Figure 2. Three stages of natural history of T1D.Table 2. Disease specificity of GAD autoantibodies.
Figure 2. Three stages of natural history of T1D.Table 2. Disease specificity of GAD autoantibodies.Subject Prevalence Healthy control <1% Acute-onset type 1 diabetes (at onset) 60–80% Fulminant type 1 diabetes 5–9% LADA (SPIDDM) 100% Type 2 diabetes (diet/OHA) 4–5% Polyglandular autoimmune syndrome, type 1 30–40% Polyglandular autoimmune syndrome, type 2 30–50% Autoimmune thyroid disease 6–8% Stiff-person syndrome 60–70% LADA, latent-autoimmune diabetes in adults; SPIDDM, slowly progressive insulin-dependent diabetes; OHA, oral hypoglycemic agents.6. Role of Anti-Islet Autoantibodies in the Diagnosis of T1D
In this section, we discuss the key considerations when diagnosing T1D using anti-islet autoantibodies. First, because distinguishing IAA from insulin antibodies produced by exogenous insulin injection is challenging, we considered patients positive for IAA if antibodies to insulin were present in insulin-naïve patients or those within 2 weeks of initiating insulin therapy. Our study using sera obtained within 2 weeks after onset revealed that the prevalence of IAA, GADA, IA-2A, and ZnT8A in patients with acute-onset T1D was 58, 80, 66, and 63%, respectively (Figure 3A). Similarly, the prevalence of these autoantibodies in SPIDDM patients was 41, 83, 27, and 27%, respectively (Figure 3B). Thus, a combinatorial analysis of the four autoantibodies indicated that 96% of acute-onset T1D and 93% of SPIDDM were immune-mediated T1D, while approximately 5% were classified as idiopathic T1D [35]. These data are consistent with previous reports from studies conducted in countries with Caucasoid populations [10][36][37][38][10,36,37,38]. In contrast, the majority of patients with fulminant T1D were negative for anti-islet autoantibodies (Figure 3C) and are classified into idiopathic T1D, suggesting that the mechanism of β-cell destruction in fulminant T1D may differ from acute-onset T1D and SPIDDM.In Japan, medical insurance guidelines instruct physicians to measure GADA first when T1D is suspected. However, about 10% of non-fulminant T1D patients are positive for other anti-islet autoantibodies without GADA. Therefore, measuring multiple autoantibodies is crucial for the accurate diagnosis of immune-mediated T1D.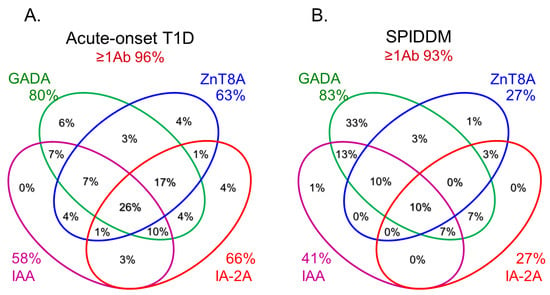
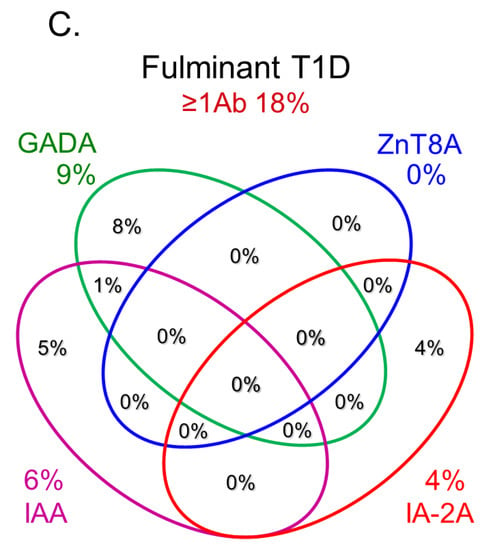 Figure 3.Combinatorial analysis of anti-islet autoantibodies in patients with acute-onset T1D (A), SPIDDM (B), and fulminant T1D (C
Figure 3.Combinatorial analysis of anti-islet autoantibodies in patients with acute-onset T1D (A), SPIDDM (B), and fulminant T1D (C7. Epitopes for Anti-Islet Autoantibodies and Their Clinical Relevance
7.1. Insulin Autoantibodies
Fewer epitope analysis studies have been conducted on IAA compared to those of GADA and IA-2A. In an earlier study, the significance of amino acid A13 of the A-chain for the binding of IAA in T1D was demonstrated [39]. Furthermore, it was revealed that conformational epitope spanning amino acid residues A8–A13 on the A-chain and B1–B3 on the B-chain was the major binding site for IAA and was disease-associated. Another epitope analysis using a recombinant Fab of the insulin-specific monoclonal antibody reported that the IAA epitope was located in the A-chain residues A8–A10 [40,41]. However, radioimmunoassay for IAA does not distinguish between epitopes on the insulin molecule bound by IAA from insulin antibodies induced by exogenous insulin injection [38]. Therefore, Devendra and coworkers used the random phage-displayed peptide library and serum obtained from an IAA-positive T1D patient and an insulin-treated insulin autoimmune syndrome patient to examine the difference in epitopes bound by both antibodies [42]. As a result, they identified the two phagotopes (phage that carry peptides that mimic
epitopes), designated IAS-9 and IDD-10, which were able to discriminate between diabetesassociated and non-diabetes associated insulin antibodies. This suggests that phage display technology could potentially be exploited to develop an IAA-specific RIA.
7.2. GAD Autoantibodies
GADA is present in 70–80% of prediabetic relatives and new-onset patients with T1D [30,35]. The major antigenic region of GADA has been determined using truncated peptide or chimeric proteins of GAD65 and GAD67 to maintain the conformational structure, as previous studies reported that GADA in patients with T1D recognizes the conformational structure of the GAD molecule [43]. Moreover, it has been reported that disease-associated GADA is directed against GAD65, and humoral immune reactivity to GAD67 is likely to be cross-reactive to GAD65 [44,45]. Figure 4A shows a schematic representation of the GAD65 and the localization of GADA epitopes in T1D. Previous
studies have demonstrated that GADA in T1D patients recognizes disease-specific GAD65 epitopes, located at the middle and C-terminal regions of GAD65 [46,47]. However, these studies may have also detected epitopes that cross-react with the GAD67 autoantibody. To detect GAD65 autoantibody-specific epitopes, we performed a competitive radioimmunoassay
with recombinant GAD67 protein using a series of GAD65/GAD67 chimeric constructs, and identified the autoantibody epitopes in the N-terminal region (amino acids 1–244; N), the middle domain (amino acids 245–359; E1 and amino acids 360–442; E3), and the C-terminal region (amino acids 443–585; E2) of GAD65 [48]. Although GADA epitope specificities remain relatively stable after the clinical onset of T1D, it has been reported that in genetically predisposed subjects, GADA is initially generated against the middle and C-terminal regions of GAD65. Furthermore, the autoimmune response may undergo intramolecular epitope spreading toward epitopes on the N-terminus and further epitopes located in the middle [49].
7.3. IA-2 Autoantibodies
As shown in Figure 4B, this protein is 979 amino acids long and comprises of a luminal domain (amino acids 27–576), transmembrane domain (amino acids 577–600), and cytoplasmic domain (amino acids 601–979). Although the PTP core sequence is found at amino acids 907–917 in the cytoplasmic domain, the expressed recombinant protein does not exhibit protein tyrosine phosphatase activity. Studies evaluating the biological properties of IA-2 have demonstrated its role as an important regulator of dense core vesicle number as well as glucose-induced and basal insulin secretion [11, 13].IA-2A is present in 60-70% of prediabetic relatives and new-onset patients with T1D. We and others have analyzed IA-2A epitopes recognized by diabetic sera using a series of IA-2 fragments or IA-2/phogrin chimeric proteins, and found that the major epitopes are localized in the cytoplasmic domain [51]. Approximately 95% of T1D patients and prediabetic relatives who are IA-2A positive recognize the PTP-like domain (amino acids 687-979), whereas only 5% of sera react with the luminal domain [51, 52]. Furthermore, our binding and competition analysis using multiple IA-2/phogrin chimeric constructs demonstrated that a major unique epitope for IA-2A is localized to amino acids 762-887. A conformational epitope associated with the C-terminal 31 amino acids of IA-2 is recognized by one-third of sera, and a minor epitope is located on amino acids 601-762 of IA-2. Notably, intramolecular epitope spreading was found for relatives of T1D patients who later progressed to T1D. However, relatives who remained nondiabetic exhibited a decrease in the number of recognized epitopes. These studies are consistent with the hypothesis that IA-2 may be recognized as a consequence of β-cell destruction [33].
Another important epitope has been mapped in the juxta-membrane domain of IA-2 (amino acids 601-629; IA-2JM). Our data demonstrated that the age of disease onset in patients with IA-2JMA only was significantly higher than that in patients who reacted with the PTP-like domain, suggesting that autoantibody recognition of IA-2 epitopes in autoimmune diabetes is associated with the age of disease onset, which may reflect the intensity of the β-cell destruction process [53].
7.4. ZnT8 autoantibodies
In 2007, Sladek and coworkers identified four loci containing variants that confer T2D risk through a genome-wide association study, including a non-synonymous polymorphism in the ZnT8 gene (SLC30A8), rs13266634 (C/T), which causes an R325W modification in the protein sequence [54]. In the same year, Hutton and coworkers discovered ZnT8 as a major autoantigen in T1D, and ZnT8A has been recognized as one of the four major anti-islet autoantibodies [23].
ZnT8A are present in 50-60% of prediabetic relatives and new-onset patients with T1D. As shown in Figure 4C, ZnT8 is a 369-amino acid polytopic transmembrane protein with cytoplasmic N- and C- terminal tails. It has been reported that ZnT8A recognizes 101 amino acids localized in the cytoplasmic C-terminal region. In particular, the amino acid residue 325 (R325W) defined by the SLC30A8 polymorphism is critical for humoral autoimmunity to this autoantigen, and binding of ZnT8A against two isotypes (ZnT8-325R, ZnT8-325W) depends on the patient's SLC30A8 genotype [55, 56]. Consequently, heterozygotes with the CT genotype respond to both ZnT8-325R and ZnT8-325W, while CC and TT homozygotes respond exclusively to ZnT8-325R or ZnT8-325W, respectively. Thus, individuals respond to endogenous ZnT8 protein determined by their own genome, and therefore, the current ZnT8A assay, therefore, uses a hybrid protein of two ZnT8 isotypes as antigens.
Furthermore, Wenzlau and coworkers identified that residues 332R, 333E, 336K, and 340K contribute to a conformational ZnT8A epitope independent of residue 325 by comparing human and mouse chimeric ZnT8 proteins [57], suggesting that this epitope may add to the diagnostic utility of measuring ZnT8A.
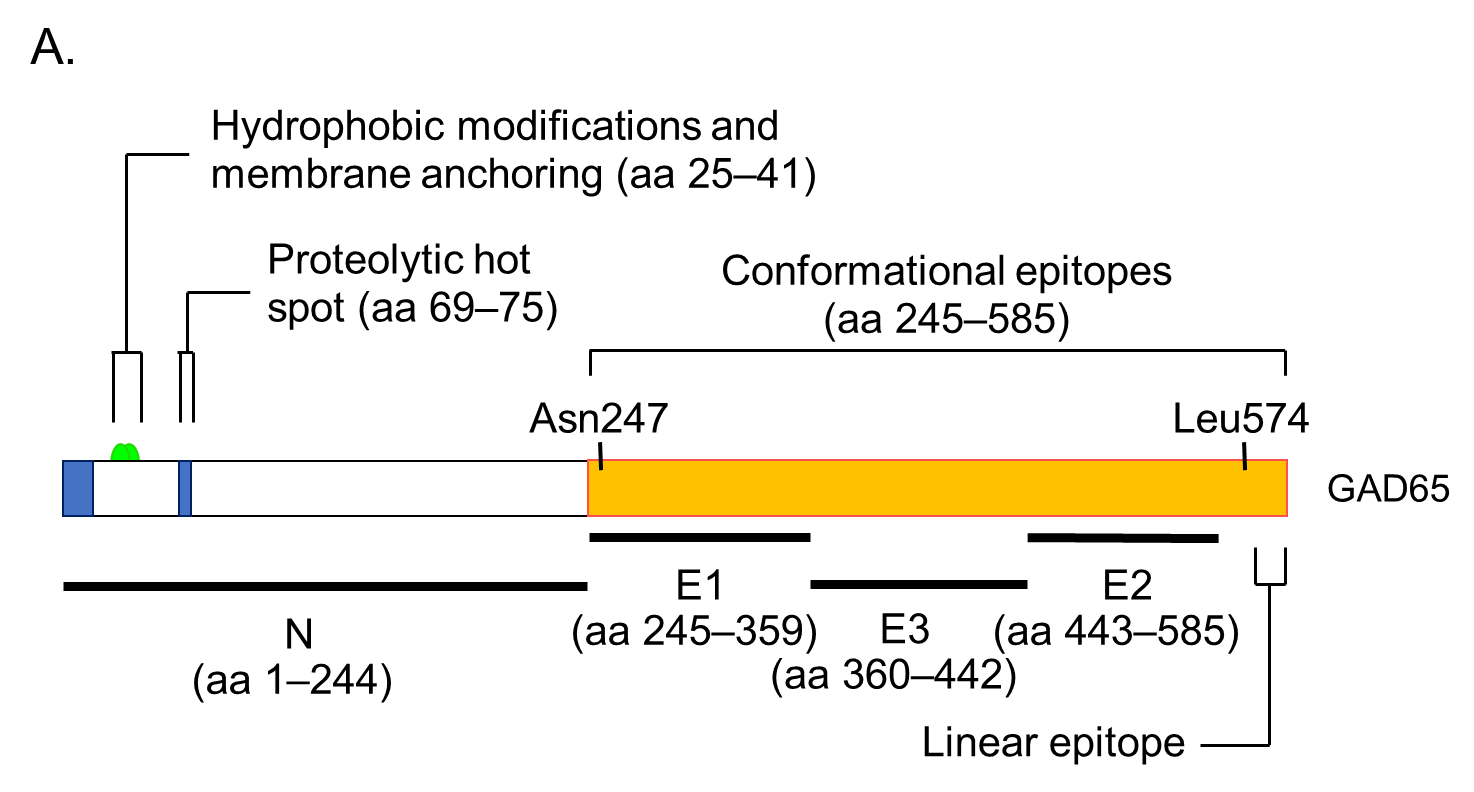
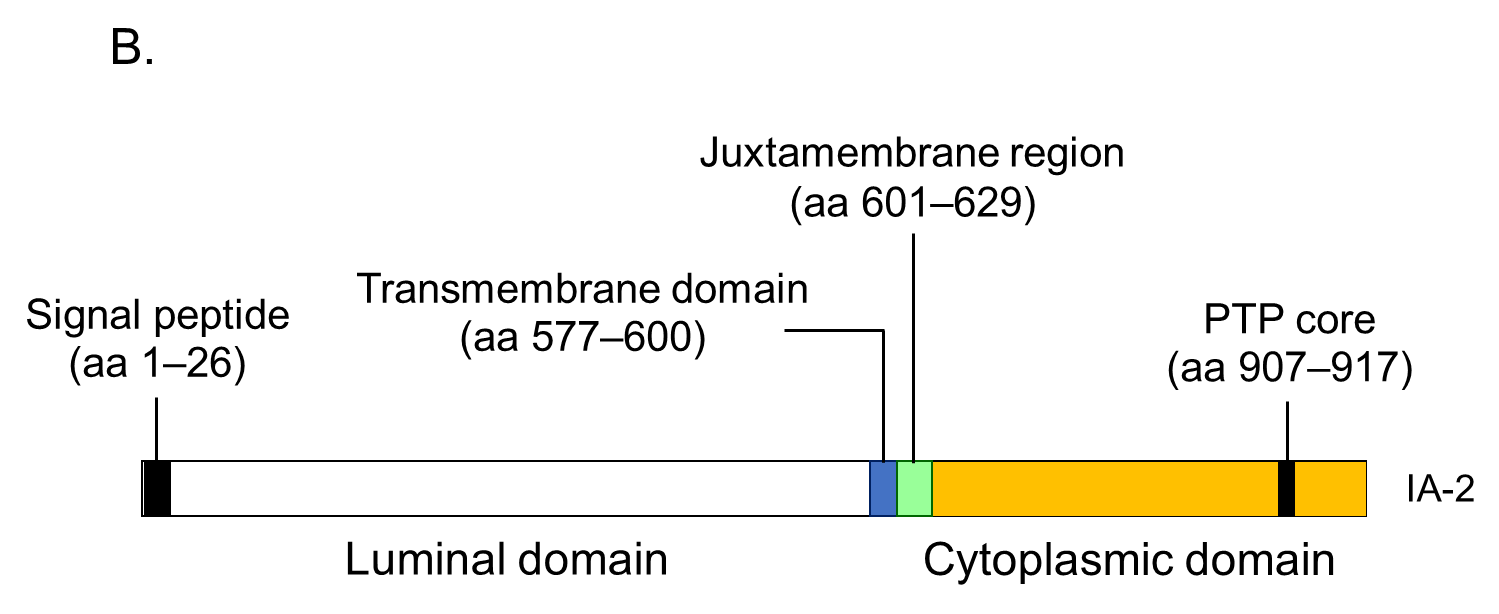
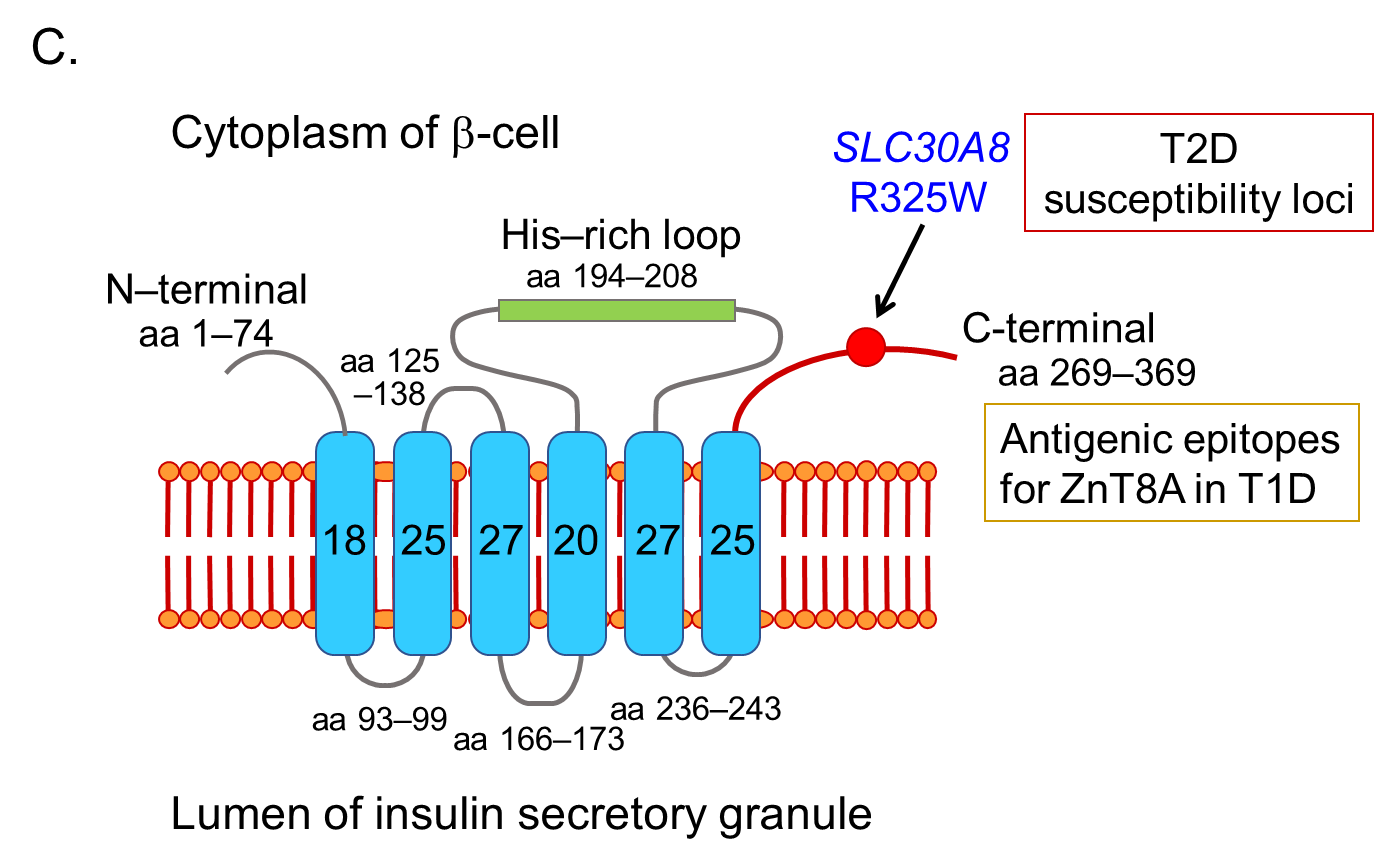
Figure 4. Illustration of antigenic epitopes recognized by T1D sera in GAD65 (A), IA-2 (B), and ZnT8 (C) proteins.
7.5. Other anti-islet autoantibodies
Other anti-islet autoantibodies include autoantibodies against GM2-1 ganglioside, HSP60, GLUT2, tetraspanin-7, and ICA12/SOX13 (Table 1). Among these, the epitopes of GM2-1 autoantibodies and GLUT2 autoantibodies have not been analyzed so far. It has been reported that HSP60 autoantibodies recognized two epitope regions on HSP60 (amino acids 394-413 and amino acids 435-454). The first region similar to the sequence found in GAD, whereas the second one overlaps with p277 T-cell epitope to a large extent [58]. Using a series of overlapping peptide fragments, Eugster and coworkers mapped map autoepitopes recognized by tetraspsnin-7 autoantibodies and found that autoantibody epitopes lie predominantly within the first and third cytoplasmic domains of the protein. Further characterization of autoantibody binding to mutated constructs revealed that epitopes lie within a relatively short (20-amino acid) region represented by at least two of the three cytoplasmic domains, providing further evidence of the importance of protein conformation in antibody binding [59]. Furthermore, epitope mapping of ICA12/SOX13 autoantibodies using several truncated fragments of SOX13 suggests that autoantibodies are directed to at least two epitopes, one that requires amino acids 66-604, and a second confined within amino acids 327-604 [22].
8. Prediction of Future Insulin Deficiency in Patients with SPIDDM (LADA)
SPIDDM (also known as LADA) is characterized by the presence of anti-islet autoantibodies and a gradual decline in insulin secretory capacity. The Immunology of Diabetes Society defined LADA as follows; 1) onset of diabetes >35 years, 2) positive test for at least one of the known anti-islet autoantibodies, and 3) requirement of insulin treatment >6 months after the diagnosis of diabetes [60]. LADA encompasses anti-islet autoantibody-positive diabetic patients in both insulin-dependent and non-insulin-dependent states, which is nearly identical to SPIDDM. According to the recently revised diagnostic criteria for SPIDDM, the interval from diabetes diagnosis to the requirement of insulin treatment is >3 months [61]. Additionally, patients with exhausted endogenous insulin secretion (Fasting C-peptide <0.6ng/ml) at the last observed time point are defined as “SPIDDM (definite)”. In contrast, anti-islet autoantibody-positive patients in non-insulin-dependent state are classified as "SPIDDM (probable)" (Table 3). Using this diagnostic criterion, measuring anti-islet autoantibodies other than GADA results in an approximately 3-fold increase in the incidence of SPIDDM among non-insulin-treated diabetic patients compared with measuring GADA alone (2.0-2.4% vs. 7-8%) [62-64]. Indeed, in the Nagasaki Autoimmune Diabetes Intervention/Prevention Study, the prevalence of anti-islet autoantibodies other than GADA in insulin naïve adult-onset diabetes was 8.6%, which is 2.6-fold compared to that of GADA (3.3%) (Figure 5). Since this subtype of T1D is generally indistinguishable from T2D at the time of diagnosis, measuring anti-islet autoantibodies is crucial for early diagnosis and appropriate treatment of SPIDDM (LADA).
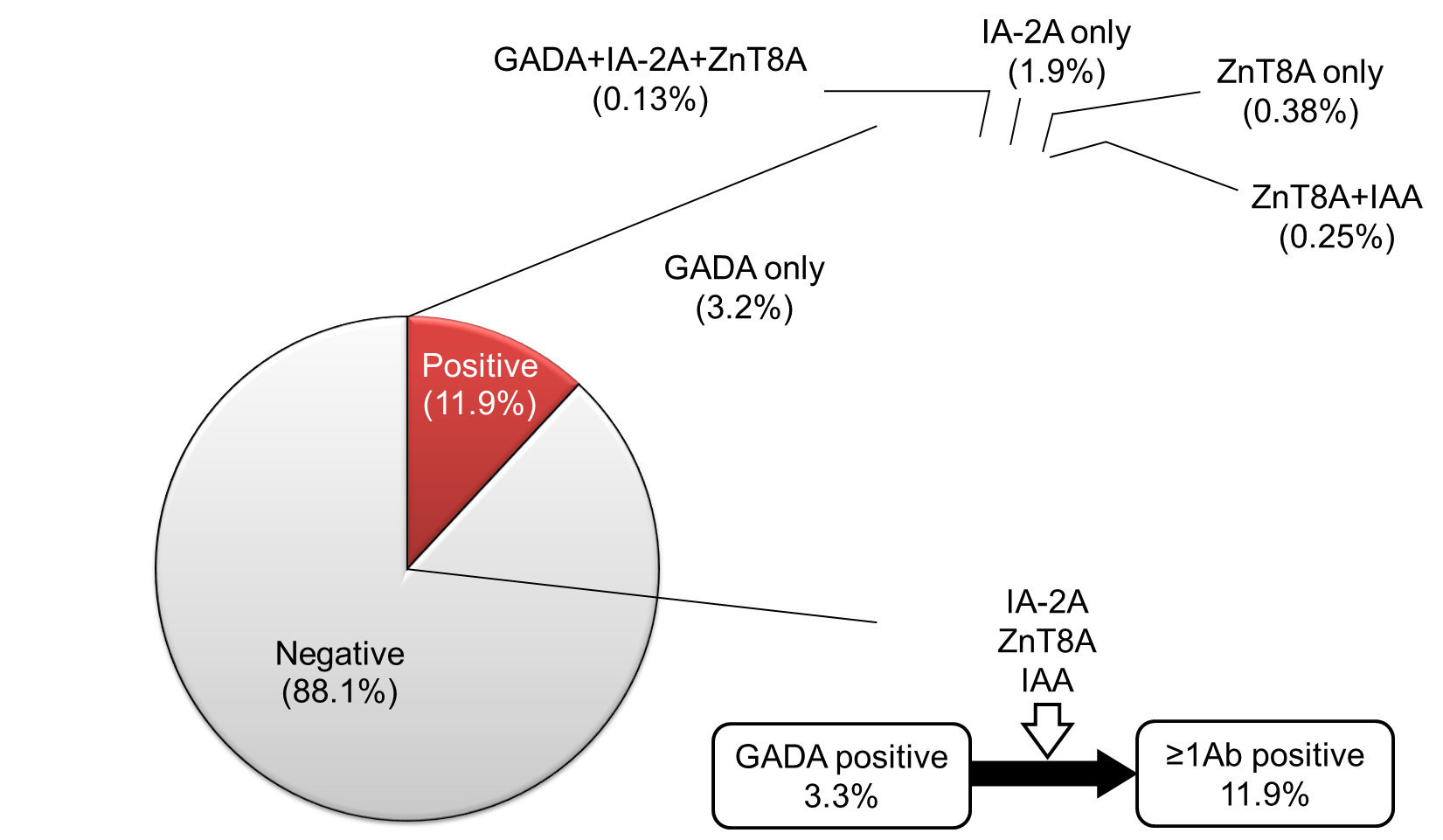
Figure 5. Prevalence of GADA, IA-2A, ZnT8A, and IAA in 788 insulin naïve adult-onset patients with diabetes
Anti-islet autoantibody positivity, especially ICA and GADA, is predictive for progression to a future insulin-dependent state after the diagnosis of diabetes. For example, the UKPDS (United Kingdom Prospective Diabetes Study) found that at least 50% of LADA patients required insulin treatment 6 years post-diagnosis [65]. However, not all SPIDDM (LADA) patients required insulin treatment, even after 10 years from diagnosis.
According to a nationwide survey [66] conducted by the Japan Diabetes Society, the predictors of progression to insulin dependent state include (1) age of onset ≤ 47 years, (2) period until GADA positive detection ≤ 5 years, (3) GADA titer (RIA method) ≥ 13.6 U/ml, and (4) fasting C-peptide ≤ 0.65 ng/mL. Additionally, the number of positive anti-islet autoantibodies and GADA epitope recognition are also important for prediction. To identify the predictive markers for early insulin requirement in non-insulin-dependent SPIDDM (probable), we evaluated IAA, IA-2A, and ZnT8A along with GADA-specific epitope recognition in 47 GADA-positive diabetic patients [63]. Among these patients, 38% had one or more of IAA, IA-2A, or ZnT8A and 15% had two or more of these autoantibodies. A high GADA titer (≥ 10U/mL), the presence of GADA-E1, and the presence of one or more among IAA, IA-2A, or ZnT8A at diagnosis marked the risk for early insulin therapy requirement. Furthermore, multiple anti-islet autoantibodies were the most relevant risk factor for the insulin requirement (odds ratio 13.77; 95% CI 2.77-68.45; P<0.001) in a multivariate logistic regression analysis. Therefore, measuring anti-islet autoantibodies other than GADA and ICA is essential for predicting the progression risk of SPIDDM (LADA) patients.
9. Conclusions
This article focused on reviewing the current understanding of anti-islet autoantibodies in T1D. The clinical utilities of anti-islet autoantibodies in patients with diabetes include diagnosis (immune-mediated or idiopathic), prediction (progressor or non-progressor) and understanding of pathophysiology (insulitis-specific or nonspecific phenomenon) (Figure 6). Since the autoantibody level of anti-islet autoantibodies decreases with disease duration and can become negative, it is essential to measure them early in the onset of T1D for accurate diagnosis. SPIDDM or LADA is often indistinguishable from T2D; therefore, earlier measurement of anti-islet autoantibodies is of great clinical importance for early diagnosis and appropriate treatment. In addition to the anti-islet autoantibody profiles, age of onset and genetic risk score should also be considered for risk triage. Furthermore, the development of a high-throughput assay to detect epitope-specific or immunoglobulin isotype-specific autoantibodies should warrant accurate diagnosis and prediction of autoimmune disorders. Besides, the new type of autoantibody assays, which can simultaneously measure multiple autoantibodies, have the advantages of high sensitivity and specificity, and the ability to measure a large number of samples, making it suitable for large-scale population screening of T1D.
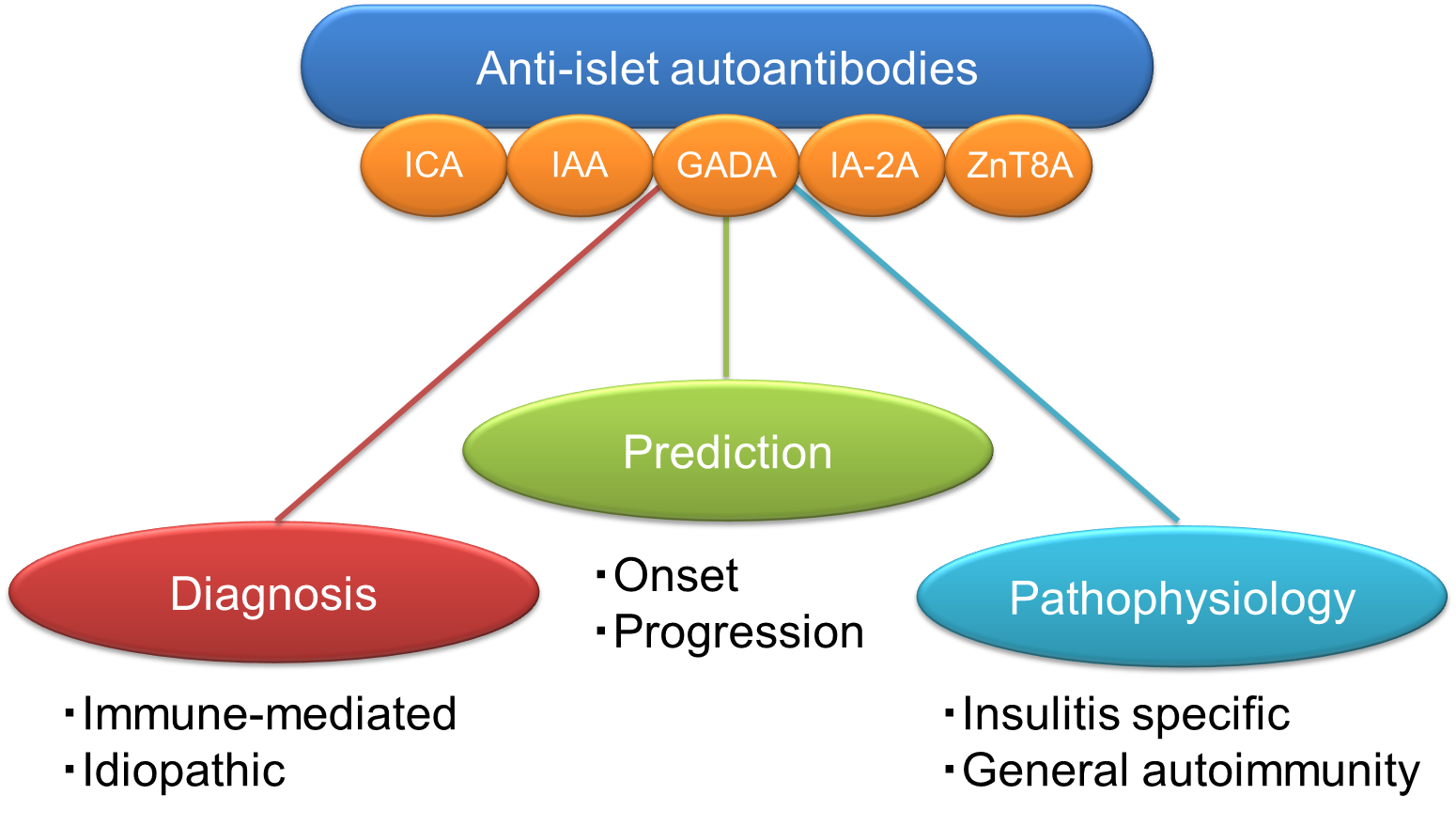
Figure 7. Clinical utilities of anti-islet autoantibodies in patients with diabetes
References
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, on behalf of the American Diabetes Association. Classification and diagnosis of diabetes: Standards of care in diabetes-2023. Diabetes Care 46(Supplement_1): S19-S40, 2023
- Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 17:150-161, 2021
- Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 23:1516-26, 2000
- Kawasaki E, Matsuura N, Eguchi K. Type 1 diabetes in Japan. Diabetologia. 49:828-836, 2006
- Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 2(7892):1279-1283, 1974
- Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark Å. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 298(5870):167-169, 1982
- Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 222(4630):1337-9, 1983
- Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 347(6289):151-156, 1990
- Christgau S, Schierbeck H, Aanstoot HJ, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S. Pancreatic beta cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem. 266:21257-21264, 1991
- Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 13:505-14, 1994
- Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci USA. 102:8704-8709, 2005
- Kawasaki E, Hutton JC, Eisenbarth GS. Molecular cloning and characterization of the human transmembrane protein tyrosine phosphatase homologue, phogrin, an autoantigen of type 1 diabetes. Biochem Biophys Res Commun. 227:440-447, 1996
- Cai T, Hirai H, Zhang G, Zhang M, Takahashi N, Kasai H, Satin LS, Leapman RD, Notkins AL. Deletion of IA-2 and/or IA-2β in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia. 54:2347-2357, 2011
- Aguilar-Diosdado M, Parkinson D, Corbett JA, Kwon G, Marshall CA, Gingerich RL, Santiago JV, McDaniel ML. Potential autoantigens in IDDM. Expression of carboxypeptidase-H and insulin but not glutamate decarboxylase on the β-cell surface. Diabetes. 43:418-425, 1994
- Spitzenberger F, Pietropaolo S, Verkade P, Habermann B, Lacas-Gervais S, Mziaut H, Pietropaolo M, Solimena M. Islet cell autoantigen of 69 kDa is an arfaptin-related protein associated with the Golgi complex of insulinoma INS-1 cells. J Biol Chem. 278:26166-26173, 2003
- Dwivedi OP, Lehtovirta M, Hastoy B, Chandra V, Krentz NAJ, Kleiner S, Jain D, Richard AM, Abaitua F, Beer NL, Grotz A, Prasad RB, Hansson O, Ahlqvist E, Krus U, Artner I, Suoranta A, Gomez D, Baras A, Champon B, Payne AJ, Moralli D, Thomsen SK, Kramer P, Spiliotis I, Ramracheya R, Chabosseau P, Theodoulou A, Cheung R, van de Bunt M, Flannick J, Trombetta M, Bonora E, Wolheim CB, Sarelin L, Bonadonna RC, Rorsman P, Davies B, Brosnan J, McCarthy MI, Otonkoski T, Lagerstedt JO, Rutter GA, Gromada J, Gloyn AL, Tuomi T, Groop L. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat Genet 51, 1596-1606, 2019
- Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58, 2070-2083, 2009
- Dotta F, Previti M, Neerman-Arbez M, Dionisi S, Cucinotta D, Lenti L, Di Mario U, Halban PA. The GM2-1 ganglioside islet autoantigen in insulin-dependent diabetes mellitus is expressed in secretory granules and is not β-cell specific. Endocrinology. 139:316-319, 1998
- Ozawa Y, Kasuga A, Nomaguchi H, Maruyama T, Kasatani T, Shimada A, Takei I, Miyazaki J, Saruta T. Detection of autoantibodies to the pancreatic islet heat shock protein 60 in insulin-dependent diabetes mellitus. J Autoimmun. 9:517-524, 1996
- Inman LR, McAllister CT, Chen L, Hughes S, Newgard CB, Kettman JR, Unger RH, Johnson JH. Autoantibodies to the GLUT-2 glucose transporter of β cells in insulin-dependent diabetes mellitus of recent onset. Proc Natl Acad Sci USA. 90:1281-1284, 1993
- Walther D, Eugster A, Jergens S, Gavrisan A, Weinzierl C, Telieps T, Winkler C, Ziegler AG, Bonifacio E. Tetraspanin 7 autoantibodies in type 1 diabetes. Diabetologia. 59:1973-1976, 2016
- Kasimiotis H, Myers MA, Argentaro A, Mertin S, Fida S, Ferraro T, Olsson J, Rowley MJ, Harley VR. Sex-determining region Y-related protein SOX13 is a diabetes autoantigen expressed in pancreatic islets. Diabetes. 49:555-561, 2000
- Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 104:17040-17045, 2007
- Fousteri G, Ippolitoa E, Ahmedb R, Hamad ARA. Beta-cell specific autoantibodies: Are they just an indicator of type 1 diabetes? Curr Diabetes Rev. 13: 322-329, 2017
- Mallone R, Brezar V. To B or not to B: (anti)bodies of evidence on the crime scene of type 1 diabetes? Diabetes 60:2020-2022, 2011
- Pinto AI, Smith J, Kissack MR, Hogg KG, Green EA. Thymic B cell-mediated attack of thymic stroma precedes type 1 diabetes development. Front Immunol 9:1281, 2018
- Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 38:1964-1974, 2015
- Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309: 2473-2479, 2013
- Krischer JP; Type 1 Diabetes TrialNet Study Group. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 56: 1919-1924, 2013
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS.Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 45:926-933, 1996
- Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 81(12):4264-4267, 1996
- Nakamura K, Kawasaki E, Abiru N, Jo O, Fukushima K, Satoh T, Kuriya G, Kobayashi M, Kuwahara H, Yamasaki H, Ide T, Eguchi K. Trajectories of anti-islet autoantibodies before development of type 1 diabetes in interferon-treated hepatitis C patients. Case reports and a literature review. Endocr J. 57:947-951, 2010
- Kawasaki E, Yu L, Rewers MJ, Hutton JC, Eisenbarth GS. Definition of multiple ICA512/phogrin autoantibody epitopes and detection of intramolecular epitope spreading in relatives of patients with type 1 diabetes. Diabetes. 47:733-742, 1998
- Kawasaki E, Yasui J, Tsurumaru M, Takashima H, Ikeoka T, Mori F, Akazawa S, Ueki I, Kobayashi M, Kuwahara H, Abiru N, Yamasaki H, Kawakami A. Sequential elevation of autoantibodies to thyroglobulin and glutamic acid decarboxylase in type 1 diabetes. World J Diabetes. 4:227-30, 2013
- Kawasaki E, Nakamura K, Kuriya G, Satoh T, Kobayashi M, Kuwahara H, Abiru N, Yamasaki H, Matsuura N, Miura J, Uchigata Y, Eguchi K.Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin Immunol. 138:146-153, 2011
- Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52:1881-1888, 2009
- Wenzlau JM, Frisch LM, Hutton JC, Fain PR, Davidson HW. Changes in zinc transporter 8 autoantibodies following type 1 diabetes onset: The type 1 diabetes genetics consortium autoantibody workshop. Diabetes Care 38(Suppl. 2):S14–S20, 2015
- Vermeulen I, Weets I, Asanghanwa M, Ruige J, Van Gaal L, Mathieu C, Keymeulen B, Lampasona V, Wenzlau JM, Hutton JC, Pipeleers DG, Gorus FK; Belgian Diabetes Registry. Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care. 34:1760-1765, 2011
- Castaño L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes. 42:1202-1209, 1993
- Padoa CJ, Crowther NJ, Thomas JW, Hall TR, Bekris LM, Torn C, Landin-Olsson M, Ortqvist E, Palmer JP, Lernmark A, Hampe CS. Epitope analysis of insulin autoantibodies using recombinant Fab. Clin Exp Immunol. 140:564-571, 2005
- Hall TR, Thomas JW, Padoa CJ, Torn C, Landin-Olsson M, Ortqvist E, Hampe CS. Longitudinal epitope analysis of insulin-binding antibodies in type 1 diabetes. Clin Exp Immunol. 146:9-14, 2006
- Devendra D, Galloway TS, Horton SJ, Evenden A, Keller U, Wilkin TJ. The use of phage display to distinguish insulin autoantibody (IAA) from insulin antibody (IA) idiotypes. Diabetologia. 46:802-809, 2003
- Tree TI, Morgenthaler NG, Duhindan N, Hicks KE, Madec AM, Scherbaum WA, Banga JP. Two amino acids in glutamic acid decarboxylase act in concert for maintenance of conformational determinants recognised by type 1 diabetic autoantibodies. Diabetologia 43: 881-889, 2000
- Kawasaki E, Yano M, Abiru N, Akazawa S, Nagataki S. Detection of recombinant GAD65 and GAD67 antibodies using a simple radioimmunoassay. Diabetes Res Clin Pract. 32:61-69, 1996
- Jayakrishnan B, Hoke DE, Langendorf CG, Buckle AM, Rowley MJ. An analysis of the cross-reactivity of autoantibodies to GAD65 and GAD67 in diabetes. PLoS ONE 6: e18411, 2011
- Daw K, Powers AC. Two distinct glutamic acid decarboxylase auto-antibody specificities in IDDM target different epitopes. Diabetes 44:216-220, 1995
- Hampe CS, Hammerle LP, Bekris L, Ortqvist E, Kockum I, Rolandsson O, Landin-Olsson M, Törn C, Persson B, Lernmark A. Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab. 85:4671-4679, 2000
- Kawasaki E, Abiru N, Ide A, Sun F, Fukushima T, Takahashi R, Kuwahara H, Fujita N, Kita A, Oshima K, Uotani S, Yamasaki H, Yamaguchi Y, Eguchi K. Epitope analysis of GAD65 autoantibodies in Japanese patients with autoimmune diabetes. Ann N Y Acad Sci. 1005:440-448, 2003
- Schlosser M, Banga JP, Madec AM, Binder KA, Strebelow M, Rjasanowski I, Wassmuth R, Gilliam LK, Luo D, Hampe CS. Dynamic changes of GAD65 autoantibody epitope specificities in individuals at risk of developing type 1 diabetes. Diabetologia 48: 922-930, 2005
- Rabin DU, Pleasic SM, Palmer-Crocker R, Shapiro JA. Cloning and expression of IDDM-specific human autoantigens. Diabetes. 41:183-186, 1992
- Kawasaki E, Yu L, Gianani R, Verge CF, Babu S, Bonifacio E, Eisenbarth GS. Evaluation of islet cell antigen (ICA) 512/IA-2 autoantibody radioassays using overlapping ICA512/IA-2 constructs. J Clin Endocrinol Metab. 82:375-380, 1997
- Acevedo-Calado M, James EA, Morran MP, Pietropaolo SL, Ouyang Q, Arribas-Layton D, Songini M, Liguori M, Casu A, Auchus RJ, Huang S, Yu L, Michels A, Gianani R, Pietropaolo M. Identification of unique antigenic determinants in the amino terminus of IA-2 (ICA512) in childhood and adult autoimmune diabetes: New biomarker development. Diabetes Care. 40:561-568, 2017
- Kawasaki E, Sera Y, Fujita N, Yamauchi M, Ozaki M, Abe T, Yamakawa K, Uotani S, Takino H, Yamasaki H, Yamaguchi Y, Uchigata Y, Matsuura N, Eguchi K. Association between IA-2 autoantibody epitope specificities and age of onset in Japanese patients with autoimmune diabetes. J Autoimmun. 17:323-331, 2001
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445(7130):881-885, 2007
- Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC. A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes. 57:2693-2697, 2008
- Kawasaki E, Uga M, Nakamura K, Kuriya G, Satoh T, Fujishima K, Ozaki M, Abiru N, Yamasaki H, Wenzlau JM, Davidson HW, Hutton JC, Eguchi K. Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia. 51:2299-2302, 2008
- Wenzlau JM, Frisch LM, Hutton JC, Davidson HW. Mapping of conformational autoantibody epitopes in ZnT8. Diabetes Metab Res Rev. 27(8):883-886, 2011
- Horváth L, Cervenak L, Oroszlán M, Prohászka Z, Uray K, Hudecz F, Baranyi E, Madácsy L, Singh M, Romics L, Füst G, Pánczél P. Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett 80:155-162, 2002
- Eugster A, Kraus G, Lidzba V, Müller D, Jolink M, Ziegler AG, Bonifacio E. Cytoplasmic ends of tetraspanin 7 harbour epitopes recognised by autoantibodies in type 1 diabetes. Diabetologia 62:805-810, 2019
- Buzzetti R, Tuomi T, Mauricio D, Pietropaolo M, Zhou Z, Pozzilli P, Leslie RD. Management of latent autoimmune diabetes in adults: A consensus statement from an international expert panel. Diabetes 69:2037-2047, 2020
- Shimada A, Kawasaki E, Abiru N, Awata T, Oikawa Y, Osawa H, Kajio H, Ozawa J, Takahashi K, Chujo D, Noso S, Fukui T, Miura J, Yasuda K, Yasuda H, Imagawa A, Ikegami H. New diagnostic criteria (2023) of slowly progressive type 1 diabetes (SPIDDM) -Report from Committee of type 1 diabetes in Japan Diabetes Society-. J Japan Diab Soc, in press (in Japanese)
- Kasuga A, Maruyama T, Nakamoto S, Ozawa Y, Suzuki Y, Saruta T. High-titer autoantibodies against glutamic acid decarboxylase plus autoantibodies against insulin and IA-2 predicts insulin requirement in adult diabetic patients. J Autoimmun 12: 131-135, 1999
- Takino H, Yamasaki H, Abiru N, Sera Y, Abe T, Kawasaki E, Yamaguchi Y, Eguchi K, Kanazawa Y, Nagataki S. Antibodies to GAD in Japanese patients classified as Type 2 diabetes at diagnosis. High titre of GADAb is a predictive marker for early insulin treatment--report of west Japan (Kyushu, Yamaguchi, Osaka) study for GAD Ab(+) diabetes. Diabet Med 19: 730-734, 2002
- Kawasaki E, Nakamura K, Kuriya G, Satoh T, Kuwahara H, Kobayashi M, Abiru N, Yamasaki H, Eguchi K. Autoantibodies to insulin, insulinoma-associated antigen-2, and zinc transporter 8 improve the prediction of early insulin requirement in adult-onset autoimmune diabetes. J Clin Endocrinol Metab 95: 707-713, 2010
- Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR, Shattock M, Bottazzo GF, Holman R. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 350:1288-1293, 1997
- Yasui J, Kawasaki E, Tanaka S, Awata T, Ikegami H, Imagawa A, Uchigata Y, Osawa H, Kajio H, Kawabata Y, Shimada A, Takahashi K, Yasuda K, Yasuda H, Hanafusa T, Kobayashi T; Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Clinical and genetic characteristics of non-insulin-requiring glutamic acid decarboxylase (GAD) autoantibody-positive diabetes: A nationwide survey in Japan. PLoS One. 11:e0155643, 2016
