Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gheorghe Ilia | -- | 2718 | 2023-06-28 08:25:04 | | | |
| 2 | Rita Xu | Meta information modification | 2718 | 2023-06-28 08:36:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ilia, G.; Simulescu, V.; Plesu, N.; Chiriac, V.; Merghes, P. Wittig Reactions under Sonication Conditions. Encyclopedia. Available online: https://encyclopedia.pub/entry/46159 (accessed on 08 February 2026).
Ilia G, Simulescu V, Plesu N, Chiriac V, Merghes P. Wittig Reactions under Sonication Conditions. Encyclopedia. Available at: https://encyclopedia.pub/entry/46159. Accessed February 08, 2026.
Ilia, Gheorghe, Vasile Simulescu, Nicoleta Plesu, Vlad Chiriac, Petru Merghes. "Wittig Reactions under Sonication Conditions" Encyclopedia, https://encyclopedia.pub/entry/46159 (accessed February 08, 2026).
Ilia, G., Simulescu, V., Plesu, N., Chiriac, V., & Merghes, P. (2023, June 28). Wittig Reactions under Sonication Conditions. In Encyclopedia. https://encyclopedia.pub/entry/46159
Ilia, Gheorghe, et al. "Wittig Reactions under Sonication Conditions." Encyclopedia. Web. 28 June, 2023.
Copy Citation
Carbonyl olefinations are among the most important organic syntheses that form C=C bonds, as they usually have high yields and in addition offer excellent stereoselectivity. Due to these advantages, carbonyl olefinations have important pharmaceutical and industrial applications. These reactions contain an additional step of an α-functionalized carbanion to an aldehyde or ketone to produce alkenes, but syntheses performed using metal carbene complexes are also known. The Wittig reaction is an example of carbonyl olefination, one of the best ways to synthesize alkenes.
Wittig reaction
Wittig–Horner synthesis
ultrasound
1. Introduction
The Wittig reaction is a chemical reaction between an aldehyde or ketone and a phosphonium ylide in the presence of a base to provide two compounds: an alkene, which has the position of the double bond well specified, and phosphine oxide. Triphenyl phosphorylide is often referred to as a Wittig reagent. This reaction was discovered in 1954 [1] by Georg Wittig. He received the Nobel Prize in Chemistry in 1979 for its discovery. It is a convenient chemical reaction for the synthesis of alkenes. Usually, disubstituted (cis, trans, or 1,1-disubstituted alkenes, Figure 1) and trisubstituted alkenes can be obtained with good yields, but for tetrasubstituted alkenes the yields are lower. Wittig reactions imply the coupling between aldehydes or ketones with monosubstituted triphenylphosphonium ylides. Interest in obtaining trisubstituted or tetrasubstituted alkenes (Figure 1) has increased in recent decades because of their higher stability in comparison with monosubstituted and disubstituted ones. Furthermore, tri- and tetrasubstituted alkenes are important synthetic targets and occasionally their synthesis can be difficult due to steric issues.
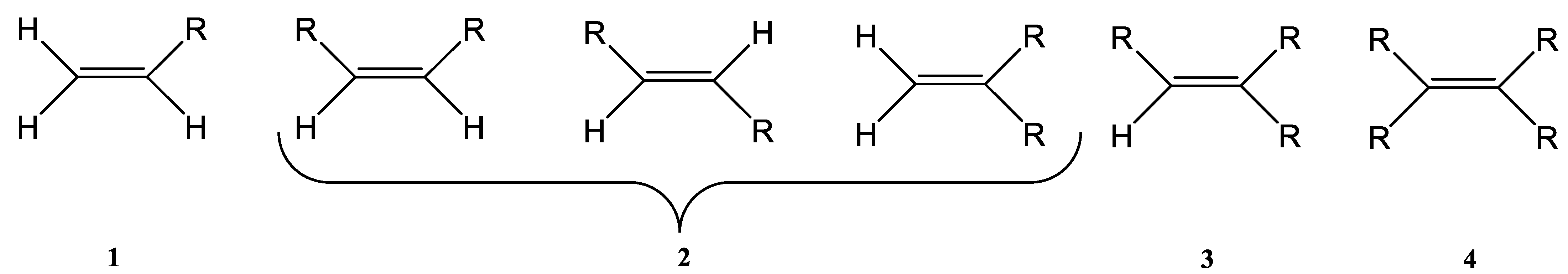
Figure 1. The structure of monosubstituted (1), disubstituted (2), trisubstituted (3), and tetrasubstituted (4) alkenes.
When Wittig synthesis is conducted in classic conditions the yields are rather low, but when using ultrasound, it was observed that the yield increases significantly, especially for obtaining tetrasubstituted alkenes. The Wittig reaction can give as products both E- and Z-alkenes or alkene derivatives. The ratio of E- and Z-isomers could be modified and controlled by changing several parameters (the electronic nature of the ylide carbanion, the presence of lithium salts, the use of phosphonium salt derived either from trialkylphosphine or triarylphosphine) [1][2][3][4][5][6][7][8][9]. The geometry of the double bond is depicted by the nature of the ylide. If the ylides are unstable (they contain an alkyl chain), the reaction product will usually be a Z-alkene. The selectivity of the reaction is moderate. On the other hand, if the ylides are stabilized (for instance by using an ester or a ketone), the product of the reaction will mostly be an E-alkene. The reaction that takes place in this case showed higher selectivity. In the case of semistabilized ylides (they contain an aryl substituent) the selectivity is rarely good and in general a mixture of E-/Z-isomers are obtained with different ratios [1][2][3][4][5][6][7][8][9].
The Wittig mechanism implies hypothetical betaine intermediates and lithium halide adducts. The stereoselectivity of the reaction depends on the formation of the covalent oxaphosphetane and on the result of the combination between steric, rehybridization effects of phosphorous, and on lithium salts [10][11]. In the first step, the alkylation of triphenylphosphine with the halogenated derivative takes place with the formation of a quaternary phosphonium salt. The deprotonation of the phosphonium salt in the presence of the base (as nBuLi, NaNH2, NaH, alkoxides, KOH (NaOH), K2CO3, tertiary amine), produces the phosphorylide (phosphorane). After elimination, if an α-bromoester is used, an enolate ion equivalent is obtained as the phosphorylide is stabilized by conjugation. If the alkylation of triphenylphosphine is undertaken with α-chloroether, a vinyl ether is formed. This undergoes acid hydrolysis. Then the reaction of phosphorylide (the nucleophilic reactant) with the carbonyl compound takes place with the formation of an oxaphosphetane intermediate with a four-atom ring, which, by elimination, leads to the formation of the two stereoisomeric alkenes. In order to obtain the more stable alkene, a phosphorylide stabilized with electron-withdrawing groups, such as vinyl, phenyl, or ester, should be used. For the less-stable alkene, a phosphorylide not stabilized by conjugation is recommended. The obtained Z:E ratios, can be better explained as a function of the level of stabilization (charge delocalization) of the ylide by a mechanism based on a cycloaddition process [12][13].
An important application of the Wittig reaction with industrial importance is the synthesis of juvenile hormones, vitamin A, β-carotene, or other aromas and flavors [14]. In Wittig syntheses, various phosphorus reagents can be used. The ‘‘classic’’ Wittig reaction uses a phosphonium ylide, the Horner–Wadsworth–Emmons reaction uses a phosphonate anion, and the Horner–Wittig uses a phosphine oxide anion [15][16]. For a nitrogen analogue of a Wittig reagent, phosphazenes (λ5 -phosphazenes, iminophosphoranes, or phosphine imines) are used in the aza-Wittig reaction [17].
The Wittig and aza-Wittig syntheses become powerful tools in organic synthesis and allow the construction of some acyclic and cyclic compounds. These reactions produce a high yield and require mild conditions (neutral solvents, absence of catalysts, generally at mild temperatures) [18][19].
The Wittig syntheses have been strongly developed and improved during recent decades. Several extensions of the method recently developed are the phospha-Wittig reaction [20], the thia-Wittig reaction [21], and the phospha-bora-Wittig reaction [22]. The phospha-bora-Wittig reaction is used for the direct preparation of phosphaalkenes, starting with aldehydes, ketones, esters, or amides. The intermediate phosphaborene reacts with the carbonyl compounds to form 1,2,3- phosphaboraoxetanes, which then undergoes thermal or Lewis acid-promoted cycloreversion, leading to the formation of phosphaalkenes [22].
2. Wittig Reactions under Sonication Conditions
Phosphorus is an essential element in life, found in many biogenic molecules (DNA, RNA, and adenosine triphosphate (ATP)). Phosphorus is also found in many other important biomolecules, including cell membrane phospholipids such as sphingomyelin. There are also many examples of active pharmaceutical ingredients (APIs) containing phosphorus with important applications in the treatment of different afflictions. Consequently, a growing interest in the synthesis of phosphorus compounds has occurred in recent decades. One of the main goals of the researchers involved in this field was to conduct these syntheses under green conditions. Therefore, green syntheses in the field of phosphorus compounds were developed significantly using ultrasounds, microwaves, green solvents, sol-gel, and others. When ultrasounds (US) or microwaves (MW) were used, an increase in the yield was observed [23][24][25][26][27][28][29].
As already mentioned, one common synthetic route for the synthesis of alkenes is the Wittig reaction (together with its modified versions, as Horner–Wittig and aza-Wittig processes). First described in 1954, the Wittig reaction [1][2][3][4] has two main steps:
- -
-
the first step is the deprotonation reaction of a phosphonium salt (5) to obtain a phosphorous ylide (6)
- -
-
the phosphorous ylide (6) reacts with a compound containing a carbonyl group, an aldehyde (7), or a ketone to give the corresponding alkene (8) and phosphine oxide (9) (Figure 2)
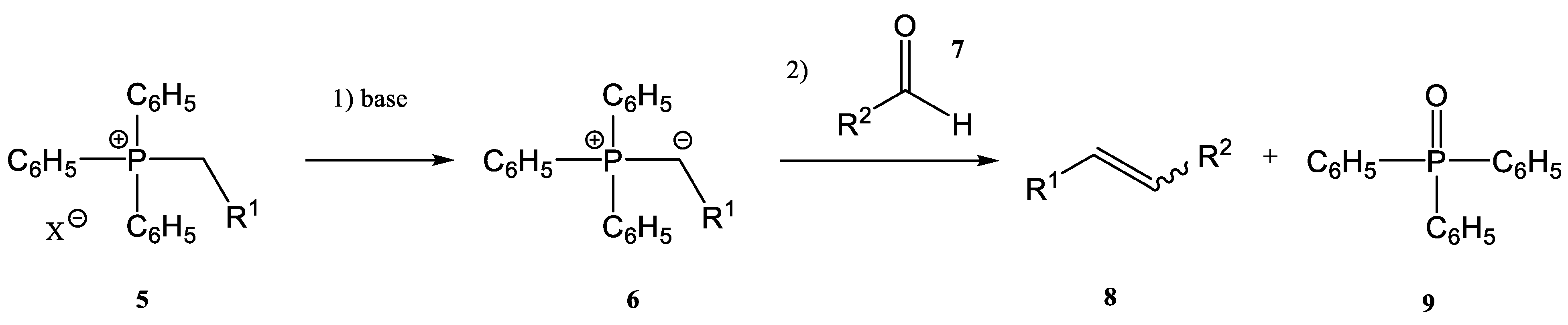 Figure 2. A general scheme of the classic Wittig reaction.
Figure 2. A general scheme of the classic Wittig reaction.
Obtaining alkenes is of great interest to researchers because alkenes could be used further as reagents for several chemical syntheses in coupling reactions and asymmetric transformations, hydrogenation, cyclopropanations, cycloadditions, epoxidations, diol formation, and so on. Most research on the Wittig reaction has been focused, especially in recent years, on triphenylphosphine-derived phosphonium salts [6]. Non-stabilized ylides generally have an alkyl group as the side chain. Under lithium-salt-free conditions, these ylides showed a significant Z-selectivity [3][4][11][12]. Ylides that were stabilized with neighboring vinyl or aryl groups showed rather low selectivity and usually lead to the formation of mixtures with E and Z isomers [6][23][30][31][32].
Triphenylphosphine-derived ylides are the most common phosphorous reagents involved in the Wittig reaction. The synthesis of E-alkenes, starting from non-stabilized or semi-stabilized triphenylphosphoranes, is not sustainable by using the standard Wittig process. In this case, if used, the Wittig synthesis requires several modifications. If the phenyl substituents from a triphenylphosphorane were replaced with short-chain alkyl substituents (i.e., ethyl, propyl) with lower hydrophobic character, a significant increase in E-alkene isomer production was observed [33][34][35]. Thus, the non-stabilized and semi-stabilized ylides derived from trialkylphosphines showed high E-isomer selectivity when used in the Wittig reaction [6]. More recently, the Wittig reaction proved to be very useful in the field of organocatalysis [36][37]. Compounds as styrenes, dienes, vinyl ethers, or allenes, synthesized by using the Wittig reaction, are used in organocatalysis [6]. Organocatalysis includes a variety of chemical transformations and is used to describe any process that is facilitated by the use of a non-metallic organic catalyst [38][39][40][41][42][43].
The Wittig synthesis could be conducted using ultrasound in order to increase the interface area (and therefore the contact) between the reagents (i.e., ylide and aldehyde or ketone). Classic Wittig methods [1][2][3][4] are usually performed at low temperatures using strong bases. The ultrasound plays the role of a solvent by increasing the mixing process. In addition, the effects of the sonic waves are higher when the reaction is performed in small channels (diameter from 10 μm to 100 μm) than in a standard flask [44].
For example, the synthesis of several cinnamic esters and their derivatives using the Wittig reaction is of great interest because such compounds are further employed in several chemical industry areas of great interest (flavors, synthetic dyes, perfumes). Moreover, cinnamic moiety is found in many biologically active molecules. From this class of compounds, 4-methoxy-ethyl-cinnamate 12 (Figure 3) is a monoamine oxidase inhibitor. Monoamine oxidase inhibitors were the first type of antidepressant medication developed [44][45].
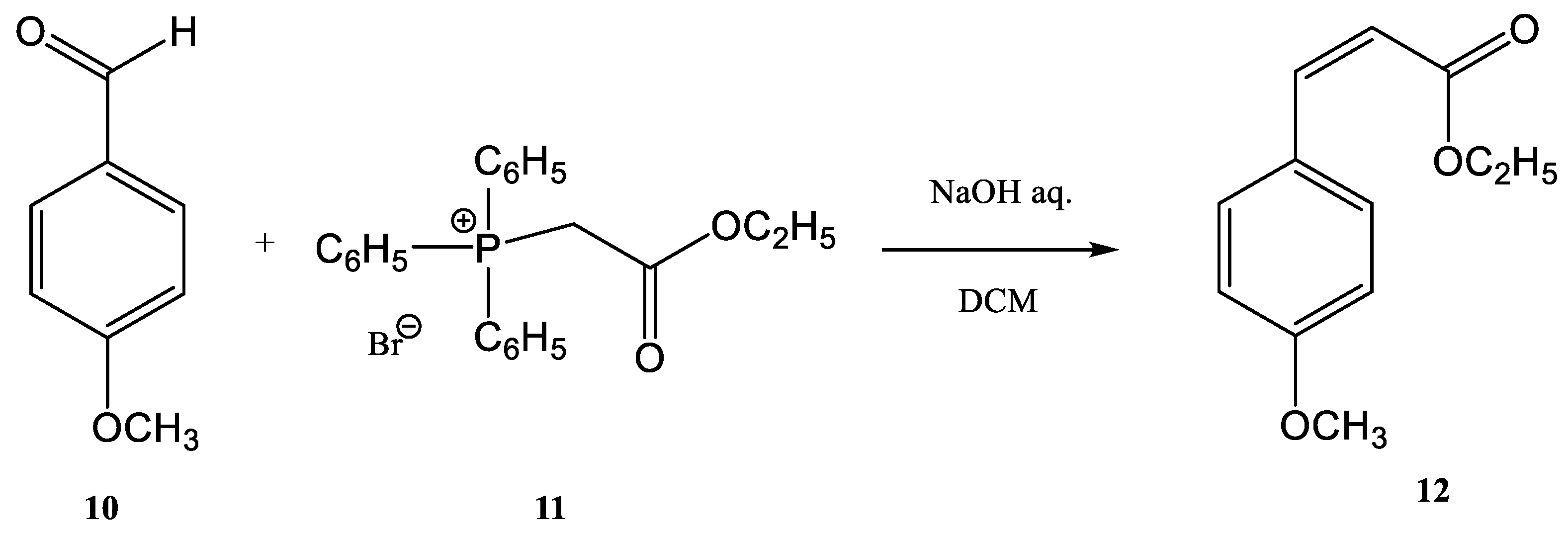
Figure 3. Synthesis of ethyl 4-methoxy-ethyl-cinnamate.
It was observed that cinnamic esters are synthesized easier, faster, and with a higher yield when the entire reactor is immersed in an ultrasonic bath. Several methods for the synthesis of this class of compounds have been published [44][45][46][47][48][49][50]. One example is the reaction of anisaldehyde 10 with (ethoxycarbonylmethyl)-triphenylphosphonium bromide 11 for obtaining ethyl 4-methoxy-ethyl-cinnamate 12 (Figure 3) [44][50].
El-Batta et al. proved that water is an effective environment for Wittig reactions by using stabilized ylides and aldehydes [51]. P-anisaldehyde slowly reacts with the ylides to obtain a mixture of E/Z cinnamic ester isomers after four hours at 20 °C, with a yield of 66% at a ratio E/Z 92/8. When the temperature of the reaction increased to 90 °C, after 30 min the yield of cinnamic ester increased to 90% without affecting or changing the E/Z-ratio. The water efficiency in the reaction environment in comparison with organic solvents is obvious, as the same reaction has been reported in refluxing DCM (four hours, 8% yield), in refluxing benzene (two days, 73% yield), and in ionic liquids at 60 °C (three days, 82% yield) [52][53][54]. On the other hand, when this synthesis procedure was performed in an ultrasonic bath, the product was obtained with a 70% yield in a shorter time. The protocol can be applied to different aldehydes, alkyl phosphonium salts, and bases for the Wittig synthesis of E-cinnamic esters under ultrasound in moderate to very good yields in the absence of any other phase transfer catalyst and in a shorter reaction time [44].
The Wittig synthesis (sometime called the Wittig olefination) is one of the most famous phosphine-based reactions. The development of continuous flow processes for Wittig olefination reactions has undergone intensive study in recent decades, with the aim of increasing the yield. The combination of flow chemistry with microwave irradiation or ultrasonication opens a new perspective from this point of view. Many pharmaceutical compounds were synthesized through the Wittig olefination [55] by using a combination of ultrasound technology and continuous flow [56][57][58]. Riccaboni et al. [44] developed a catalyst-free continuous flow biphasic system for the Wittig synthesis of disubstituted alkenes 15 (Figure 4) [59][60].
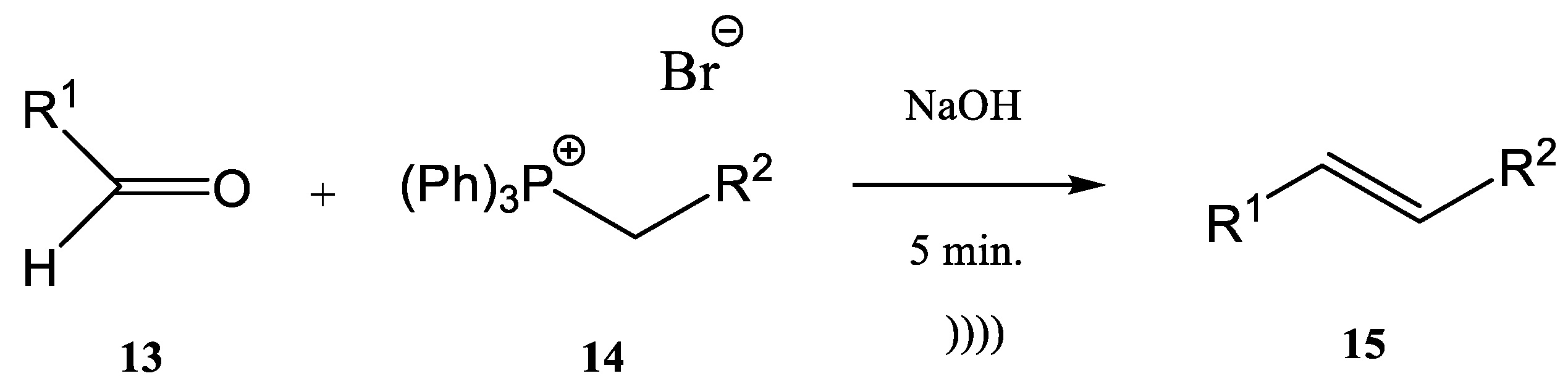
Figure 4. Ultrasound-assisted Wittig olefination in biphasic media.
The synthesis was performed starting from an aldehyde (13), triphenyl-phosphonium bromide (14), and NaOH at a ratio of the used reagents 13:14:NaOH of 1:2:5 (an excess of phosphonium bromide 14 and of NaOH was used). The reaction mixture was immersed in an ultrasonic bath for enhancing the interfacial interactions in the absence of a phase-transfer catalyst (Figure 4) [44][59][60][61].
Modest to quantitative yields were obtained at room temperature after five minutes. The authors reported the in situ preparation of the phosphonium salt 14 by mixing triphenylphosphine (PPh3) and ethyl 2-bromoacetate. When the phosphonium salt 14 was prepared in situ, the yields of the synthesis increased [24]. A similar Wittig synthesis was proposed by Krajnc et al. [62]. Benzyl-triphenyl-phosphonium bromide salt (17) and o- or p-methoxy-benzaldehydes (16) were mixed at a 1:1 eq. ratio in CH2Cl2 and injected together with an aqueous solution of 0.1 M NaOH. The corresponding stilbene derivative 18 (Figure 5) was obtained after a maximum reaction time of 9–10 min. The yields changed from 68% for o-methoxybenzaldehydes to 90% for p- methoxybenzaldehydes.
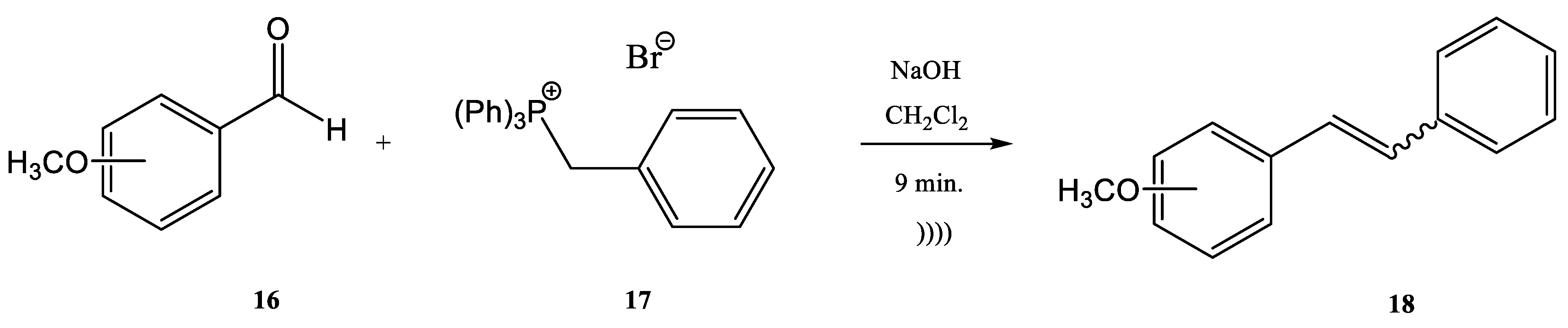
Figure 5. Continuous flow process for the synthesis of stilbene derivatives 18 by Wittig reaction with a benzyltriphenyl-phosphonium bromide salt 17 acting both as reactant and phase-transfer catalyst.
Viviano et al. synthesized different active pharmaceutical ingredients using a Wittig olefination process [63]. Starting from the aldehyde 19, different synthetic routes were used in order to synthesize the 4-aryl-3-buten-2-one intermediates 21a–c. The reaction can be conducted as a continuous flow process (Figure 6A) as follows: aldehyde 19 reacts with (acetylmethylene)triphenyl-phosphorane 20 in DMF and the products 4-aryl-3-buten-2-one intermediates 21a–c in good yields (around 98%) at 210 °C for 10 min. Then, 4-aryl-3-buten-2-ones 21a–c were further converted under pressure using a Raney-Ni catalyst through a hydrogenolysis reaction (Figure 6B) [24]. The hydrogenolysis represents a chemical reaction where a carbon–carbon or carbon–heteroatom bond is cleaved or undergoes “lysis” by hydrogen.
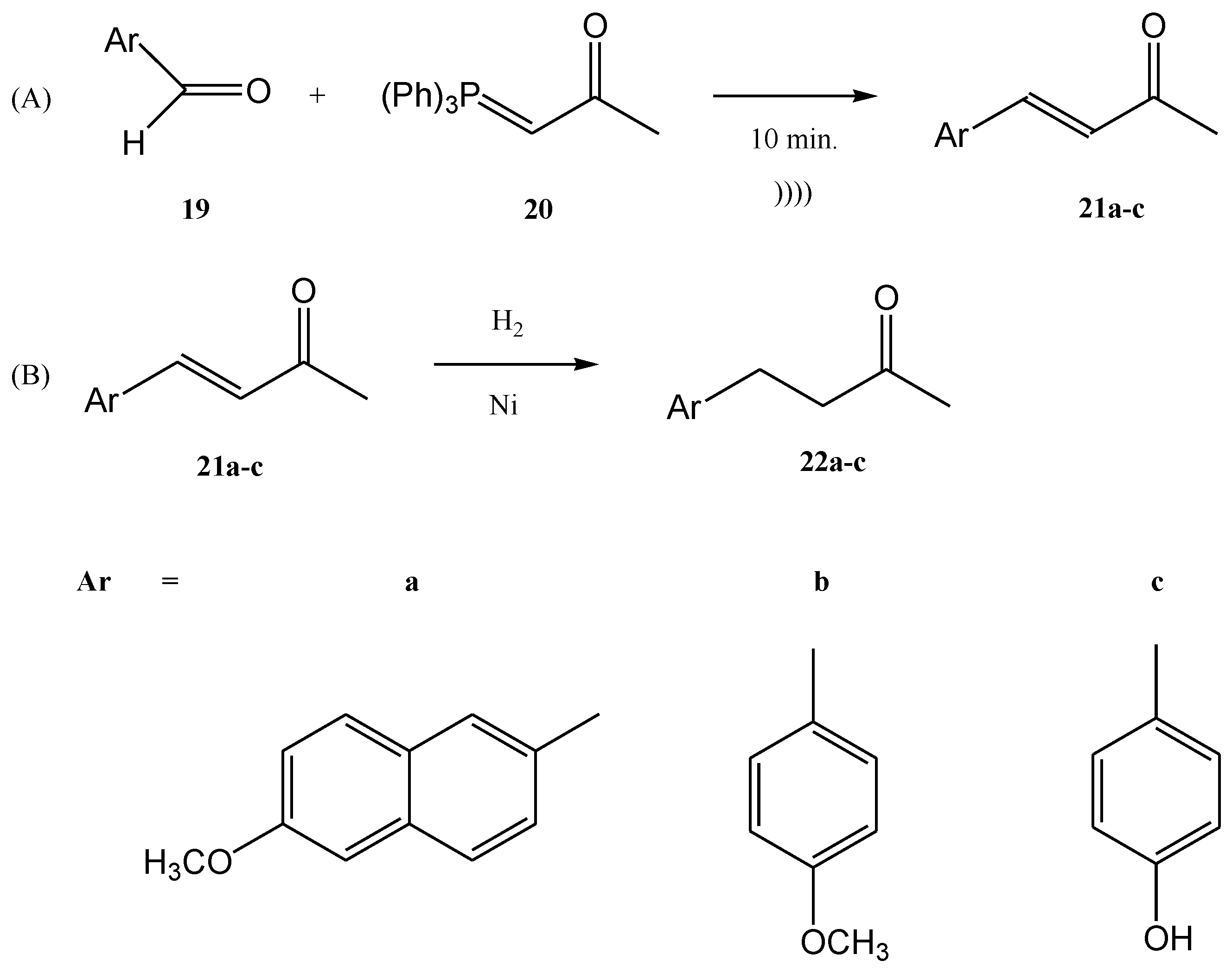
Figure 6. (A) The continuous flow Wittig olefination (B) the further reduction of the compounds 21a-c.
The hydrogenolysis reaction of the alkene 21a takes place in ethanol and the hydrogenolysis process of alkenes 21b and 21c takes place in DMF. At temperatures ranging from 20 °C to 100 °C, the final products with active pharmaceutical properties were obtained in good yields, as follows: 22a—91%, 22b—90%, 22c—94%. The compounds 22a and 22b are commonly used in cosmetics. On the other hand, the compound 22c, currently named nabumethone, is a nonsteroidal anti-inflammatory drug used to reduce pain, swelling, and joint stiffness from arthritis. Nabumethone can be used only with a doctor or pharmacist’s recommendation [63].
The previously discussed examples showed the Wittig reaction employed alone on different syntheses. Moreover, the Wittig reaction could be involved in the synthesis of phosphorus compounds in tandem with other types of chemical processes in a one-step procedure. For instance, the halogenation of an ylide and the oxidation of an alcohol with the common reagent MnO2 as the oxidant and a Wittig reaction together could be conducted in a one-step procedure using ultrasounds. In the work published by Karama et al. [64] the (carboethoxymethylene)triphenyl-phosphorane 23 reacted with a reactive alcohol (as for instance aromatic, allylic and propargylic alcohols) in the presence of N-bromosuccinimide (NBS) and manganese dioxide, in CH2Cl2 (Figure 7).
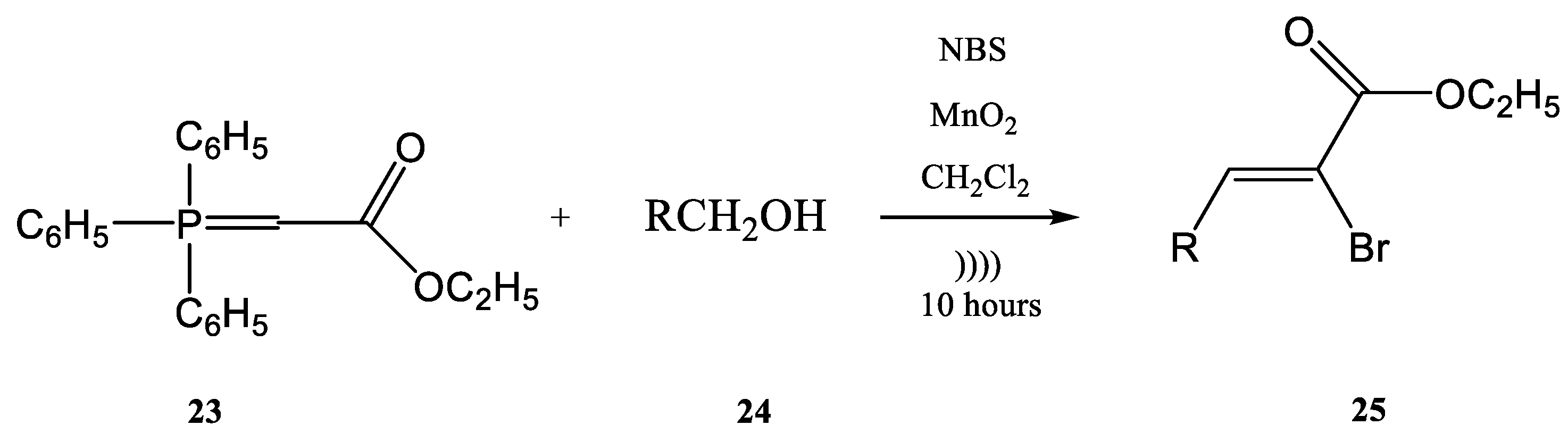
Figure 7. The tandem halogenation–oxidation–Wittig reaction in one-step, conducted under ultrasonication.
Another example of using the Wittig reaction as a green method (in this case also the use of ultrasounds) using phosphonium ylide as reagent is the work of Maity et al. [65]. The Wittig process was performed under ultrasonication, starting from aldehydes (26, 29) and ylides (27). The products 28 and 30 were further obtained with high yields as a mixture of E- and Z-isomers (E/Z = 76/24 in the case of the product 28, and 84/16 in the case of the compound 30) [65] (Figure 8a,b).
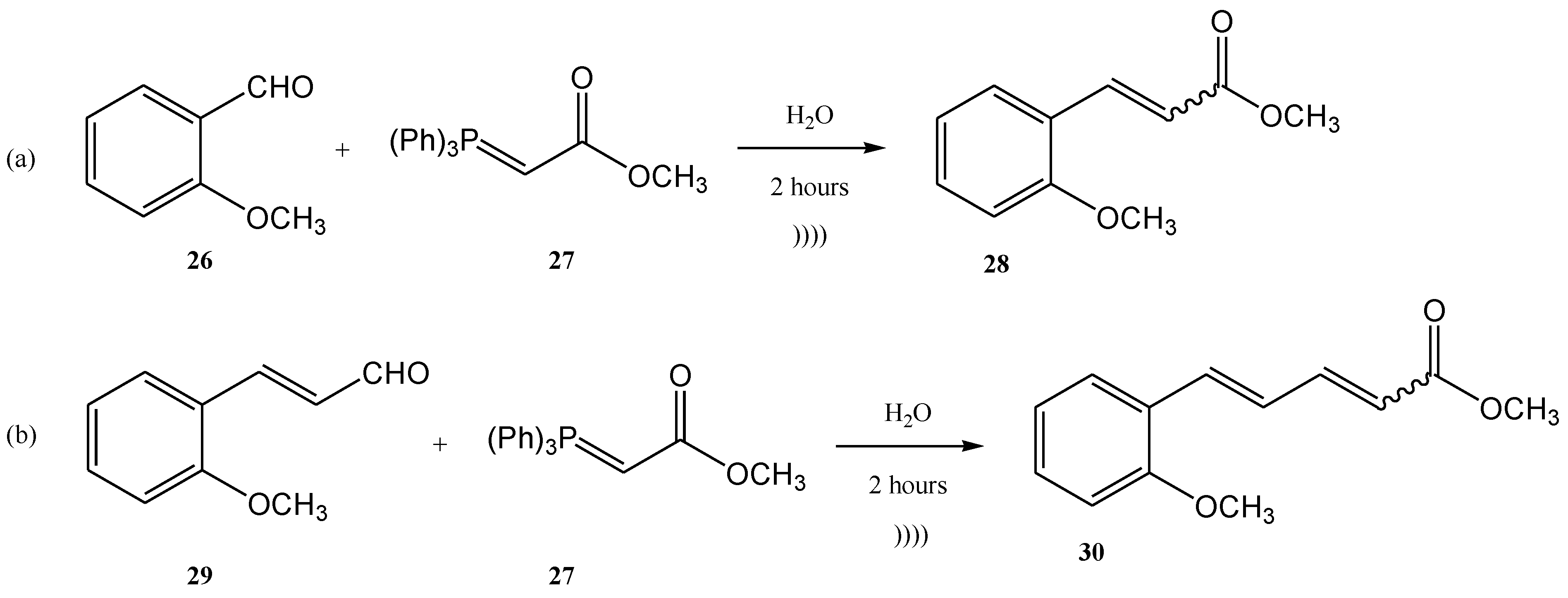
Figure 8. Examples of two Wittig reactions used as green methods, performed under ultrasound.
The ultrasound irradiation for the Wittig reaction is usually performed in a water bath of an ultrasonic cleaner with a frequency of approximately 40 KHz and a power of approximately 250 W [66]. Currently, several products of growing interest are synthesized in this way. Benzoquinones, for instance, represent an important class of biologically active compounds, which were also obtained by the Wittig reaction performed under ultrasound. [61][66] 2-methoxy-6-alkyl-1,4-benzoquinones are compounds that occur in nature (usually in plants) and most of them have significant biological activity (anti-cancer activity and 5-1ipoxygenase inhibitory activity). Lipoxygenase enzymes catalyze the deoxygenation processes of polyunsaturated fatty acids to obtain lipids. The ultrasound-assisted Wittig reaction of o-vanillin 31 with alkyltriphenyl phosphonium bromides 32 in the presence of K2CO3 leads to the formation of styrene 35 in 72–81% yields (Figure 9) [5][61].
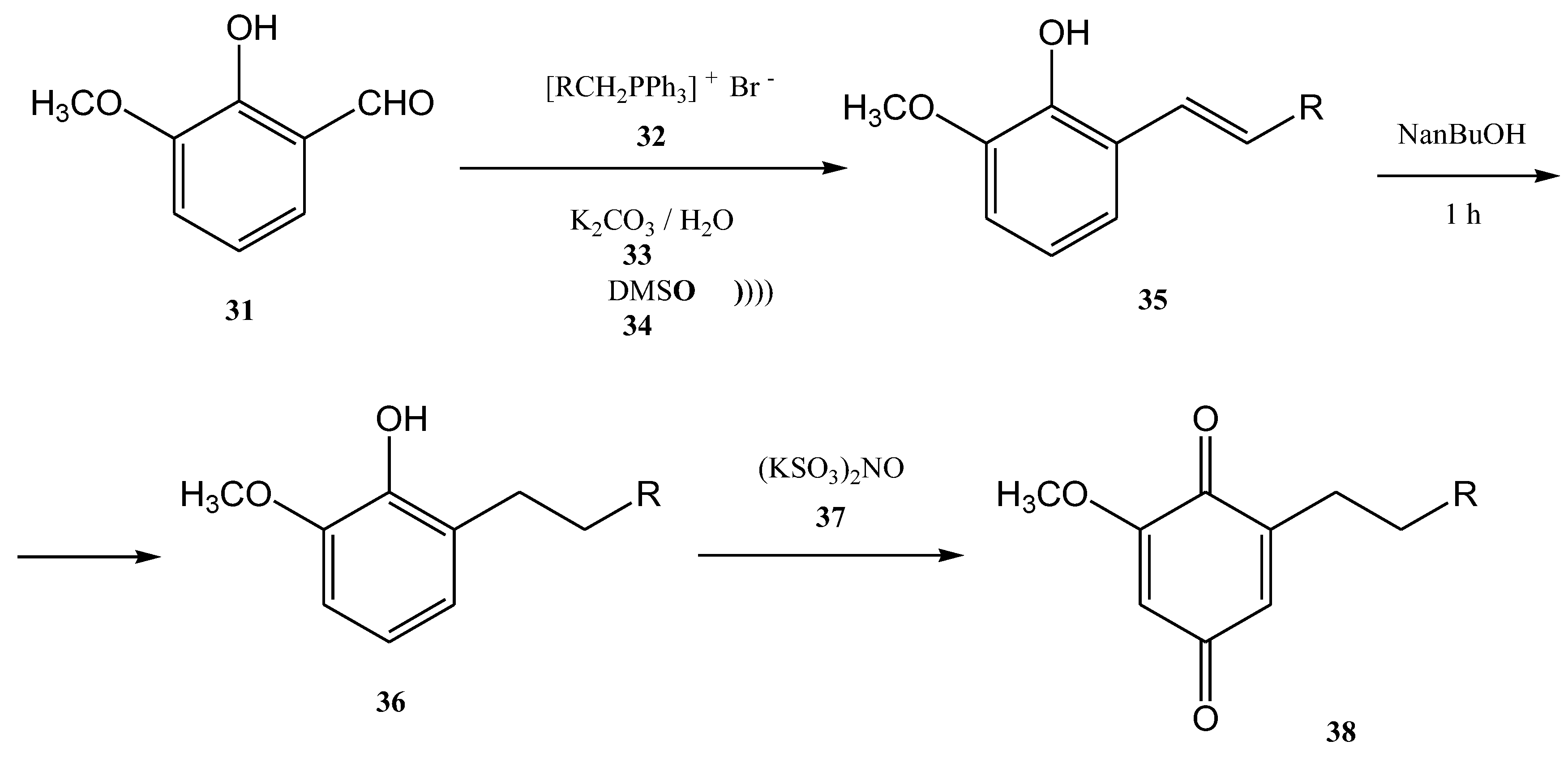
Figure 9. The Wittig synthesis of 2-methoxy-6-alkyl-1,4-benzoquinones, performed under ultrasonication.
This reaction requires a mixture of DMSO (34) and water as solvents at 90–100 °C. Then, the2-methoxy-6-alkenyl-1,4-benzoquinones can be obtained through the hydrogenolysis reaction. If the hydrogenolysis of the styrene 35 is performed directly to obtain 2-methoxy-6-alkenyl-1,4-benzoquinone, the double bond from its structure is actually reduced. Consequently, the styrene 35 was first treated with metallic sodium in n-butanol. In this way, the 2-methoxy-6-alkyl-phenols 36 were further successfully synthesized in 74–84% yields after one hour at 80–90 °C. The conjugated olefin was reduced but the isolated olefin was not affected [5][61].
This step of the synthesis was followed by the oxidation of 2-methoxy-6-alkylphenols 36 with Fremy’s salt (KSO3)2NO (37). Then, 2-methoxy-6-alkyl-1,4-benzoquinones 38 was obtained as a solid yellow product in 79–92% yields [61].
References
- Georg, W.; Ulrich, S. Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien I. Chem. Ber. 1954, 87, 1318.
- Wittig, G.; Weigmann, H.-D.; Schlosser, M. Abwandlung der Phosphylen-Methodik; zugleich III. Mitteil. über Phosphin-alkylene als olefinbildende Reagenzien. Chem. Ber. 1961, 94, 676–689.
- Wittig, G.; Haag, A. Über Phosphin-alkylene als olefinbildende Reagenzien, VIII. Allenderivate aus Ketenen. Chem. Ber. 1963, 96, 1535–1543.
- Horner, L.; Hoffmann, H.M.R.; Wippel, H.G. Phosphororganische verbindungen, XII. Phosphinoxyde als olefinierungsreagenzien. Chem. Ber. 1958, 91, 61–63.
- Horner, L.; Hoffmann, H.M.R.; Wippel, H.G.; Klahre, G. Phosphororganische verbindungen, XX. Phosphinoxyde als olefinierungsreagenzien. Chem. Ber. 1959, 92, 2499–2505.
- Nielsen, A.J. Novel Wittig and Organocatalytic Methodologies for the Synthesis of Chemotherapeutic Compounds. Ph.D. Thesis, MCmaster University, Hamilton, ON, Canada, 2019.
- Pommer, H.; Thieme, P.C. Top. Curr. Chem. Wittig Chem. 1983, 109, 165–188.
- Takeda, T. Modern Carbonyl Olefination: Methods and Applications; Wiley-VCH: Weinheim, Germany, 2016.
- Laue, T.; Plagens, A. Named Organic Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2005; p. 293.
- Maryanoff, B.; Reitz, A.; Mutter, M.; Inners, R.; Almond, H.R., Jr.; Whittle, R.; Olofson, R. Stereochemistry and Mechanism of the Wittig Reaction. Diastereomeric Reaction Intermediates and Analysis of the Reaction Course. J. Am. Chem. Soc. 1986, 108, 7664–7678.
- Reitz, A.; Nortey, S.; Jordan, A.; Mutter, M.; Maryanoff, B.E. Dramatic concentration dependence of stereochemistry in the Wittig reaction. Examination of the lithium salt effect. J. Org. Chem. 1986, 51, 3302–3308.
- Vedejs, E.; Marth, C.F. Mechanism of Wittig reaction: Evidence against betaine intermediates. J. Am. Chem. Soc. 1990, 112, 3905–3909.
- Byrne, P.; Gilheany, D.G. The modern interpretation of the Wittig reaction mechanism. Chem. Soc. Rev. 2013, 42, 6670–6696.
- Majid, M.; Vahideh, Z.; Mansoureh, D.; Manizheh, G. Applications of Wittig Reaction in the Total Synthesis of Natural Macrolides. Chem. Select 2020, 5, 9654–9690.
- Roman, D.; Sauer, M.; Beemelmanns, C. Applications of the Horner–Wadsworth–Emmons Olefination in Modern Natural Product Synthesis. Synthesis 2021, 53, 2713–2739.
- Maranescu, B.; Lupa, L.; Tara-Lunga Mihali, M.; Plesu, N.; Maranescu, V.; Visa, A. The corrosion inhibitor behavior of iron in saline solution by the action of magnesium carboxyphosphonate. Pure Appl. Chem. 2018, 90, 1713–1722.
- Palacios, F.; Alonso, C.; Aparicio, D.; Rubiales, G.; de los Santos, J.M. The aza-Wittig reaction: An efficient tool for the construction of carbon–nitrogen double bonds. Tetrahedron 2007, 63, 523–575.
- Lao, Z.; Toy, P.H. Catalytic Wittig and aza-Wittig reactions. Beilstein J. Org. Chem. 2016, 12, 2577–2587.
- Palacios, F.; Aparicio, D.; Rubiales, G.; Alonso, C.; de los Santos, J.M. Synthetic Applications of Intramolecular Aza-Wittig Reaction for the Preparation of Heterocyclic Compounds. Curr. Org. Chem. 2009, 13, 810–828.
- Smith, R.; Chen, X.; Protasiewicz, J.D. A Fluorescent (E)-Poly(p-phenylenephosphaalkene) Prepared by a Phospha-Wittig Reaction. Inorg. Chem. 2003, 42, 5468–5470.
- Chevrie, D.; Lequeux, T.; Pommelet, J.-C. Thia-Wittig-like Reactions as a New Route for the Stereoselective Synthesis of (Z)-Fluoroalkenoates. Org. Lett. 1999, 1, 1539–1541.
- Borys-Smith, A.; Rice, E.; Nichol, G.; Cowley, M.J. The Phospha-Bora-Wittig Reaction. J. Am. Chem. Soc. 2021, 143, 14065–14070.
- Ward, W.J., Jr.; McEwan, W.E. Metal ion effects in Wittig reactions. A general hypothesis for the mechanism of the Wittig reaction. J. Org. Chem. 1990, 55, 493–500.
- Morodo, R.; Bianchi, P.; Monbaliu, J.-C. Continuous Flow Organophosphorus Chemistry. Eur. J. Org. Chem. 2020, 33, 5236–5277.
- Drehe, M.; Simulescu, V.; Ilia, G. Progress in the development of flame retardants. Rev. Chem. Eng. 2008, 24, 263–302.
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153.
- Jansa, P.; Baszczyňski, O.; Procházková, E.; Dračínský, M.; Janeba, Z. Microwave-assisted hydrolysis of phosphonatediesters: An efficient protocol for the preparation of phosphonic acids. Green Chem. 2012, 14, 2282–2288.
- Bálint, E.; Tajti, Á.; Tóth, N.; Keglevich, G. Continuous Flow Alcoholysis of Dialkyl H-Phosphonates with Aliphatic Alcohols. Molecules 2018, 23, 1618.
- Peters, D.; Scheunemann, A.; Zur, C.; Miethchen, R. Fluorination of organic compounds with ultrasound. Ultrasonics 1996, 34, 543–546.
- Vedejs, E.; Peterson, M.J. Top. Stereochem; Eliel, E.L., Wilen, S.H., Eds.; Wiley: New York, NY, USA, 1994; Volume 21.
- McKenna, E.G.; Walker, B.J. The stereochemistry of Wittig reactions of ylide-anions derived from semi-stabilized phosphonium ylides. Tetrahedron Lett. 1988, 29, 485–488.
- Yamataka, H.; Nagareda, K.; Ando, K.; Hanafusa, T. Relative reactivity and stereoselectivity in the Wittig reactions of substituted benzaldehydes with benzylidenetriphenylphosphorane. J. Org. Chem. 1992, 57, 2865–2869.
- McNulty, J.; McLeod, D.; Das, P.; Zepeda-Velázquez, C. Wittig Reactions of Trialkylphosphine-derived Ylides: New Directions and Applications in Organic Synthesis. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 619–632.
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. Comprehensive Asymmetric Catalysis: Supplement 1 and 2; Springer: Berlin/Heidelberg, Germany, 2004.
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms and Structure 2007, 6th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007.
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New Strategies for Organic Catalysis: The First Highly Enantioselective Organocatalytic Diels−Alder Reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244.
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-Catalyzed Direct Asymmetric Aldol Reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396.
- MacMillan, D.W.C. The advent and development of organocatalysis. Nature 2008, 455, 304–308.
- Dondoni, A.; Massi, A. Asymmetric organocatalysis: From infancy to adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660.
- Notz, W.; Tanaka, F.; Barbas, C.F. Enamine-based organocatalysis with proline and diamines: The development of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels-alder reactions. Acc. Chem. Res. 2004, 37, 580–591.
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis—After the gold rush. Chem. Soc. Rev. 2008, 38, 2178–2189.
- Dalko, P.I. Enantioselective Organocatalysis: Reaction and Experimental Procedures; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2007.
- Vedejs, E.; Marth, C.F.; Ruggeri, R. Substituent effects and the Wittig mechanism: The case of stereospecific oxaphosphetane decomposition. J. Am. Chem. Soc. 1988, 110, 3940–3948.
- Riccaboni, M.; La Porta, E.; Martorana, A.; Attanasio, R. Effect of phase transfer chemistry, segmented fluid flow, and sonication on the synthesis of cinnamic esters. Tetrahedron 2010, 66, 4032–4039.
- Noro, T.; Miyase, T.; Kuroyanagi, M.; Ueno, A.; Fukushima, S. Monoamine oxidase inhibitor from the rhizomes of Kaempferia galanga L. Chem. Pharm. Bull. 1983, 31, 2708–2711.
- Fujimura, O.; Honma, T. Olefination of aldehydes by ethyl diazoacetate catalyzed by a ruthenium(II) complex. Tetrahedron Lett. 1998, 39, 625–626.
- Calo, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. Pd Nanoparticles Catalyzed Stereospecific Synthesis of β-Aryl Cinnamic Esters in Ionic Liquids. J. Org. Chem. 2003, 68, 2929–2933.
- Ahmed-Omer, B.; Barrow, D.; Wirth, T. Heck reactions using segmented flow conditions. Tetrahedron Lett. 2009, 50, 3352–3355.
- Schwalbe, T.; Autze, V.; Hohmann, M.; Stirner, W. Novel innovation systems for a cellular approach to continuous process chemistry from discovery to market. Org. Process Res. Dev. 2004, 8, 440–454.
- Frattini, S.; Quai, M.; Cereda, E. Kinetic study of microwave-assisted Wittig reaction of stabilized ylides with aromatic aldehydes. Tetrahedron Lett. 2001, 42, 6827–6829.
- El-Batta, A.; Jiang, C.; Zhao, W.; Annes, R.; Cooksy, A. Bergdahl, Wittig Reactions in Water Media Employing Stabilized Ylides with Aldehydes. Synthesis of α,β-Unsaturated Esters from Mixing Aldehydes, α-Bromoesters, and Ph3P in Aqueous NaHCO3. J. Org. Chem. 2007, 72, 5244–5259.
- Nonnenmacher, A.; Mayer, R.; Plieninger, H. Application of high-pressure on Wittig reactions with resonance stabilized ylides. Liebigs Ann. Chem. 1983, 23, 2135–2140.
- Boulaire, V.L.; Grèe, R. Wittig reactions in the ionic solvent . Chem. Commun. 2000, 22, 2195–2196.
- Mahboobi, S.; Sellmer, A.; Höcher, H.; Garhammer, C.; Pongratz, H.; Maier, T.; Ciossek, T.; Beckers, T. 2-Aroylindoles and 2-Aroylbenzofurans with N-Hydroxyacrylamide Substructures as a Novel Series of Rationally Designed Histone Deacetylase Inhibitors. J. Med. Chem. 2007, 50, 4405–4418.
- Rocha, D.H.A.; Pinto, D.C.G.A.; Silva, A.M.S. Applications of the Wittig Reaction on the Synthesis of Natural and Natural-Analogue Heterocyclic Compounds. Eur. J. Org. Chem. 2018, 20, 2443–2457.
- Skelton, V.; Greenway, G.M.; Haswell, S.J.; Styring, P.; Morgan, D.O.; Warrington, B.; Wong, S.Y.F. The preparation of a series of nitrostilbene ester compounds using micro reactor technology. Analyst 2001, 126, 7–10.
- Skelton, V.; Greenway, G.M.; Haswell, S.J.; Styring, P.; Morgan, D.O.; Warrington, B.H.; Wong, S.Y.F. The generation of concentration gradients using electroosmotic flow in micro reactors allowing stereoselective chemical synthesis. Analyst 2001, 126, 11–13.
- Comer, E.; Organ, M.G. A Microreactor for Microwave-Assisted Capillary (Continuous Flow) Organic Synthesis. J. Am. Chem. Soc. 2005, 127, 8160–8167.
- Rivas, D.F.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374, 70.
- Dong, Z.; Delacour, C.; Carogher, K.M.; Udepurkar, P.A.; Kuhn, S. Continuous Ultrasonic Reactors: Design, Mechanism and Application. Materials 2020, 13, 344.
- Wu, L.-Q.; Yang, C.-G.; Yang, L.-M.; Yang, L.-J. Ultrasound-assisted Wittig Reaction: A Short, Efficient Synthesis of 2-Methoxy-6-alkyl-1,4-benzoquinones. J. Chin. Chem. Soc. 2009, 56, 47–50.
- Šinkovec, E.; Krajnc, M. Phase Transfer Catalyzed Wittig Reaction in the Microtube Reactor under Liquid–Liquid Slug-Flow Pattern. Org. Process Res. Dev. 2011, 15, 817–823.
- Viviano, M.; Glasnov, T.N.; Reichart, B.; Tekautz, G.; Kappe, C.O. A Scalable Two-Step Continuous Flow Synthesis of Nabumetone and Related 4-Aryl-2-butanones. Org. Process Res. Dev. 2011, 15, 858–870.
- Karama, U.; Al-Othman, Z.; Al-Majid, A.; Almansour, A. A Facile One-Pot Synthesis of α-Bromo-α,β-unsaturated Esters from Alcohols. Molecules 2010, 15, 3276–3280.
- Maity, G.; Ghosha, A.K.; Ghosh, P.K. Green Chemistry: Synthesis of organic compounds through green approach. J. Indian Chem. Soc. 2020, 97, 2897–2902.
- Wu, L.; Yang, C.; Yang, L. Synthesis of 2-Hydroxy-5-methoxy-3-alkyl-1,4-benzoquinones. Asian J. Chem. 2009, 21, 7005–7011.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
28 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




