Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihaela Badea | -- | 2206 | 2023-06-21 19:56:29 | | | |
| 2 | Catherine Yang | Meta information modification | 2206 | 2023-06-25 04:45:48 | | | | |
| 3 | Catherine Yang | Meta information modification | 2206 | 2023-06-25 04:46:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chelmea, L.; Badea, M.; Scarneciu, I.; Moga, M.A.; Dima, L.; Restani, P.; Murdaca, C.; Ciurescu, D.; Gaman, L.E. Uric Acid Electroanalysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/45952 (accessed on 07 February 2026).
Chelmea L, Badea M, Scarneciu I, Moga MA, Dima L, Restani P, et al. Uric Acid Electroanalysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/45952. Accessed February 07, 2026.
Chelmea, Ligia, Mihaela Badea, Ioan Scarneciu, Marius Alexandru Moga, Lorena Dima, Patrizia Restani, Cecilia Murdaca, Daniel Ciurescu, Laura Elena Gaman. "Uric Acid Electroanalysis" Encyclopedia, https://encyclopedia.pub/entry/45952 (accessed February 07, 2026).
Chelmea, L., Badea, M., Scarneciu, I., Moga, M.A., Dima, L., Restani, P., Murdaca, C., Ciurescu, D., & Gaman, L.E. (2023, June 21). Uric Acid Electroanalysis. In Encyclopedia. https://encyclopedia.pub/entry/45952
Chelmea, Ligia, et al. "Uric Acid Electroanalysis." Encyclopedia. Web. 21 June, 2023.
Copy Citation
Uric acid is a metabolic product that results from degradation of purines in the liver. Usually, uric acid is identified from biological fluids, human serum and urine through conventional methods, such as spectroscopy, chromatography, electrochemistry, membrane capillary electrophoresis and spectrophotometric methods, including uricase enzymatic reactions. Importantly, uric acid determination opens the possibility of early intervention in cases of hyperuricemia and preventing the degradation of renal function.
uric acid
chemosensors
biosensors
nanocomposites
1. Introduction
From the electrochemical point of view, uric acid is a weak acid, with two-step dissociation at a pKa1 of 5.4 and a pKa2 of 9.8. In the physiological range of pH (7.35–7.45), in the extracellular compartment, uric acid is found mostly (98%) in the form of biurate (deprotonated urate anion or ionized urate), and a very small quantity (<1%) is found as undissociated uric acid [1]. However, in more acid pH media, such as urine (pH 6.5), uric acid is still found mainly as biurate (88%) but with an increased percentage as uric acid (12%) [1][2].
The physiological levels of uric acid are between 3.5 mg/dL and 7.2 mg/dL (210 μM and 430 μM) in males, and between 2.6 mg/dL and 6.0 mg/dL (155 μM and 360 μM) in premenopausal females [1]. These levels are maintained by exogenous input (diet) but mostly by endogenous formation (nucleic acid catabolism and de novo synthesis) [3].
At high levels of uric acid, hyperuricemia, the undissociated uric acid precipitates at the vascular level and biurate is implicated in kidney stones formation. This phenomenon occurs because of the low solubility (6 mg/dL or 360 µM) of uric acid, mainly in the form of monosodium urate [1].
The oxidation of uric acid starts with the formation of diimine (a) by exchanging 2e− and 2H+. The resulting diimine takes up two molecules of water and forms imine-alcohol (b) and uric acid-4,5-diol (c), successively. Ultimately, uric acid-4,5-diol is decomposed to allantoin (d) and CO2 in neutral pH (Figure 1) [4].
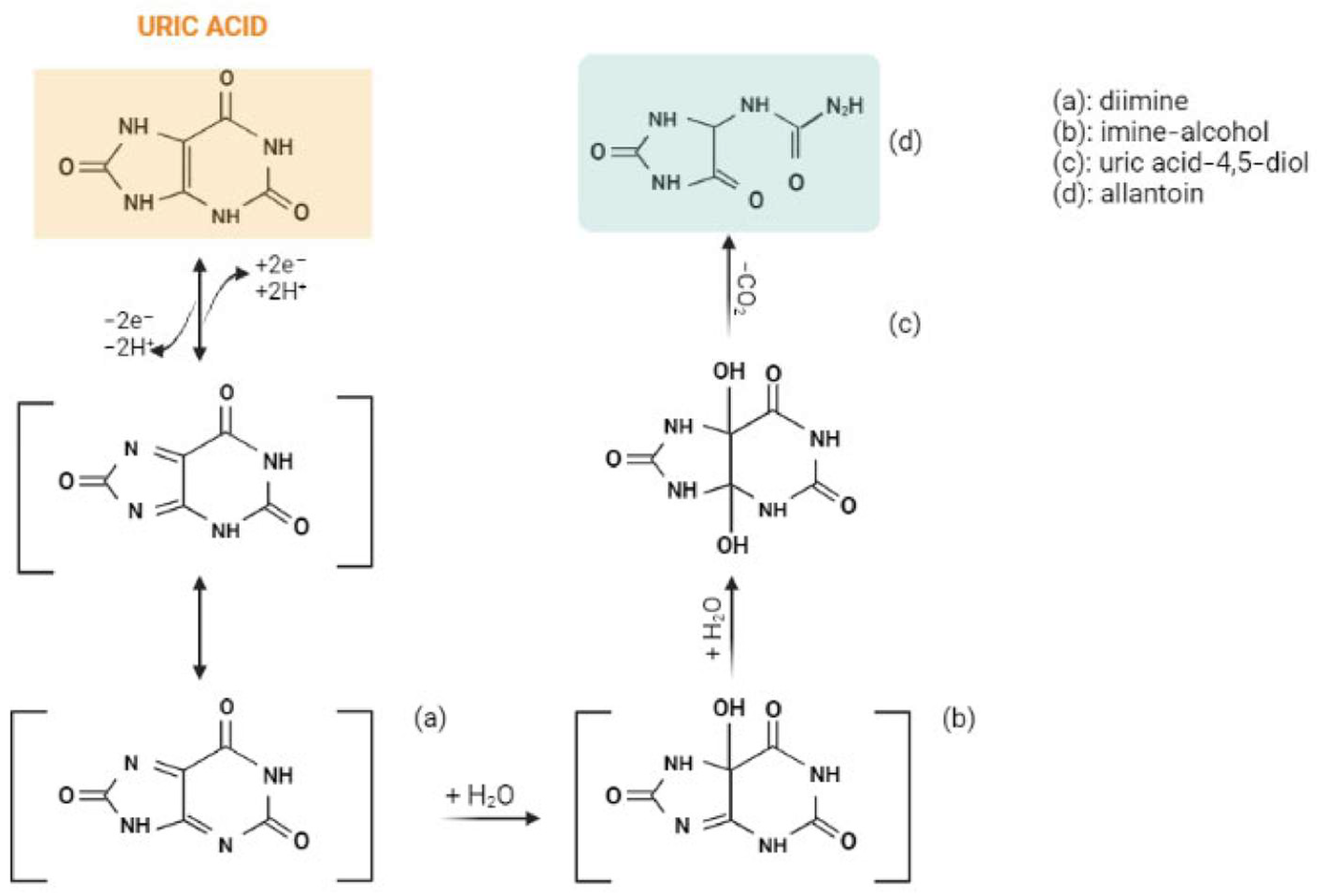
Figure 1. The electrochemical oxidation of uric acid to allantoin. Created with BioRender.com.
The oxidative properties of uric acid can be used in developing catalytic methods of detection. Thanks to the high electrochemical capacity of uric acid, for the rapid quantification of uric acid levels, scientists have developed different uric acid detection tools.
Together with uric acid, dopamine and ascorbic acid have similar oxidative behavior and coexist in urine samples [5]. Therefore, uric acid, dopamine and ascorbic acid signals can interfere with each other in the process of electrochemical detection in real samples. These three compounds have a very similar oxidation potential, so their electrochemical detection is very challenging [5] as obtaining separate voltametric peaks is the principal objective [6]. This matter has been investigated frequently for most types of electrodes, such as conventional sensors, modifiable electrodes and biosensors.
However, dopamine, uric acid and ascorbic acid have individual and cumulative importance because of their role in oxidative stress-related diseases [7]. Parkinson’s disease, most of all, lacks a rapid diagnostic method using biological markers for diagnosis of the early stages of the pathology [8] and it is an example where simultaneous detection of the three compounds may be useful [9].
Other cases in which it may be important to establish levels of uric acid and its electrochemically similar compounds, dopamine and ascorbic acid, in biological matrixes are the following: dopamine: cardiotoxicity [10], aging [11], multiple sclerosis [12], rheumatoid arthritis [12], Alzheimer’s disease [12], and Tourette [12]; uric acid: arthritis [13], gout [13], Lesch–Nyhan syndrome [13], urolithiasis [13], kidney damage [13], leukemia [14], lymphoma [14], and multiple sclerosis [15]; and ascorbic acid: high blood pressure [16], heart attack risk [16], cataracts [16], tooth decay [16], improper bone development [16], loss of appetite [16], weakened cartilage [16], skin hemorrhages [16], impaired digestion [16], septic shock [17], and diabetes mellitus [18].
2. Uric Acid Electrochemical Detection
Among the transition metal oxide-modified electrodes, ZnO NWAs/GF/GCE [19] had the best performance in terms of sensitivity, but highly selective sensors with moderately higher limits of detection included GCE/MC–GO–Fe3O4 [20], CuO/GCE [21] and RuON-GCE [22] (Table 1).
Table 1. Comparison of electrodes modified with transition metal nanoparticles for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| GCE/MC–GO–Fe3O4 1 | CV, DPV | 7.0 | UA, AA, DA, G, sucrose, L-Cys, citric acid, Fe2+, Cl−, Na+, NO3− | Human urine RR > 96% |
0.5–140 | 0.17 | [20] |
| TiO2 NPs/GCE 2 | DPV | 7.0 | UA | Human urine RR: 97–99.6% |
1–9 | 0.764 | [23] |
| PdNPs/rGO/GCE 3 | DPV | 7.2 | UA, AA, DA | Human serum RR: 96.6–108.5% |
0.3–1400 | 16.67 | [24] |
| SnO2/chitosan/GCE 4 | DPV | UA, AA, DA | Human urine RR: 97.4% |
3–200 | 1 | [25] | |
| CuO/GCE 5 | CV | 7.4 | UA, UR, lactic acid, ethanol, G, K+, Na+ | Human urine RR: 95–104% |
0.001–351,000 | 0.6 | [21] |
| RuON-GCE 6 | DPV | 7.0 | UA, E | Human urine RR: 98–101.6% |
3.0–56.6; 56.6–758.6 | 0.47 | [22] |
| MoS2 NSA/CNFs 7 | CV, DPV | 7.0 | UA, levodopa | Human urine RR: 99.7–102.6% |
1–60 | 1 | [26] |
| CuO nano-rice/GCE 8 | CV, DPV | 7.0 | UA, AA, DA, G, fructose, galactose, lactose, Na+, Cl−, K+, Ca2+, Br−, CO23−, NH4+, NO2−, NO3−, SO42−, SO32− | Human urine RR: 98.6–102.6% |
1–60 | 1.2 | [27] |
| Fe3O4@CNT-N/GCE 9 | SWV | 2.5 | UA, AA, DA | - | 25–85 | 0.47 | [28] |
| ZnO NWAs/GF/GCE 10 | DPV | 7.4 | UA, AA, DA | Human serum | 0–40 | 0.001 | [19] |
AA = ascorbic acid, Cys = cysteine, DA = dopamine, E = epinephrine, G = glucose, SWV = square wave voltammetry, UA = uric acid, UR = urea; 1 = Glassy carbon electrode based on modified methylcellulose by graphene oxide and Fe3O4 nanoparticles; 2 = Glassy carbon electrode coated with titanium dioxide nanoparticles; 3 = Palladium nanoparticles/reduced graphite oxide nanocomposite on a glassy carbon electrode; 4 = SnO2 nanoparticles/multi-walled carbon nanotubes/carbon paste electrode; 5 = Glassy carbon electrode coated with copper oxide; 6 = Glassy carbon electrode modified with ruthenium oxide nanoparticles; 7 = MoS2 nanosheet arrays/carbon nanofibers; 8 = CuO nano-rice-modified glassy carbon electrode; 9 = N-doped carbon nanotubes functionalized with Fe3O4 nanoparticles; 10 = Glassy carbon electrode modified with ZnO nanowire arrays on 3D graphene foam.
From Table 2, it can be noted that some gold-coated sensors demonstrated very good anti-interference response in real samples, including Au@Pd-RGO [29], PEI/[P2W16V2-Au/PDDA-rGO]8 [30], GO/AuNR/GCE [31], ITO-rGO-AuNPs [32], GCE-PErGO-AuNP [33], and Nafion/AuNPs/AzA/MWCNTs [34]. The Au@Pd-RGO [29] sensor had the lowest limit of detection of 0.005 μM [29] for isolated detection of uric acid.
Table 2. Comparison of gold-coated electrodes for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| GO/AuNR/GCE 1 | DPV | - | UA, AA, DA, G, UR, Mg2+ | Human urine | 10–90 | 0.4 | [31] |
| AuNPs@GO/PPy/CFP 2 | DPV | 7.0 | UA, AA, DA | Human urine RR: 96.8–109% |
2–360 | 1.68 | [35] |
| AuNPs-GO/Au-IDA 3 | CV | 7.0 | UA, AA, DA, G, E | Human urine | 2–1050 | 0.62 | [36] |
| GCE-PErGO-AuNP 4 | CV, DPV | 7.4 | UA, AA, DA | Human urine | 20–260 | 20 | [33] |
| AuRGO/GCE 5 | DPV | 7.0 | UA, AA, DA | Human serum RR: 97.5–102% |
88–53 | 1.8 | [37] |
| Au@Pd-RGO/GCE 6 | DPV | 7.0 | UA, AA, DA | Human urine RR: 97.1–102.5% |
0.02–500; 0.1–350 |
0.005; 0.02 |
[29] |
| PEI/[P2W16V2-Au/PDDA-rGO]8 7 | DPV | 7.0 | UA, AA, DA, NaCl, KCl, NH4Cl, L-Cys, L-Glu, CA, UR, G | Human urine RR: 95.2–103.1% |
0.25–1500 | 0.08 | [30] |
| rGO-PAMAM-CNT-Au 8 | DPV | 4.0 | UA, AA, DA | - | 1–114 | 0.33 | [38] |
| Naf/AuNPs/AzA/MWCNTs 9 | DPV | 7.0 | UA, AA, DA, Trp, Na+, K+, Ca2+, Mg2+, G, citric acid, tartaric acid | Human urine RR: 99.7–103% |
0.5–50 | 0.28 | [34] |
| ITO-rGO-AuNPs 10 | LSV | 8.0 | UA, AA, Cl, Na+, Ca2+ NH4+ | Human urine, milk | 10–500 | 3.6 | [39] |
| EGFET-AuE 11 | - | 7.0 | UA, AA, G, bilirubin, hemoglobin | Human urine, serum | 1–1000 | 0.5 | [15] |
AA = ascorbic acid, CA = citric acid, Cys = cysteine, DA = dopamine, E = epinephrine, G = glucose, Glu = glutamate, LSV = linear sweep voltammetry, Trp = tryptophan, UA = uric acid, UR = urea. 1 = Gold nanorod-decorated graphene oxide glassy carbon electrode; 2 = Gold nanoparticles-decorated polypyrole/graphene oxide nanosheets; 3 = Gold interdigitated microelectrodes array modified with graphene oxide and doped with gold nanoparticles; 4 = Gold nanoparticles deposition on reduced graphene oxide based on glassy carbon electrode; 5 = Reduced graphene oxide and gold nanoplates-modified glassy carbon electrode; 6 = Reduced graphene oxide-supported bimetallic, gold-palladium nanocomposites; 7 = Poly(diallyldimethylammonium chloride)-functionalized reduced graphene oxide and polyoxometalates-doped gold nanoparticles sensor; 8 = sensor based on reduced graphene oxide functionalized by poly(amido-amine), multi-walled carbon nanotubes and gold nanoparticles; 9 = Nafion-based electrode modified with Azure A-coated carbon nanotubes coated with gold nanoparticles; 10 = polyethylene terephthalate substrate coated with indium tin oxide and combined with reduced graphene oxide and gold nanoparticles; 11 = 11-(ferrocenyl)undecanethiol-modified gold electrode.
Regarding the chemosensors, most of the sensors are highly sensitive for detection of uric acid (Table 3). Some sensors demonstrated good sensitivity and higher specificity, including ZIF-11/GCE [13], boron-doped diamond electrode [40], over-oxidized poly(3,4-ethylenedioxythiophene) nanofibers/PGE [41], and tosyl surface carbon nanoparticles/GCE [42]. Table 4 shows a large variety of biosensors with good and high selectivity for uric acid from real samples: zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase [43], poly(brilliant green) and poly(thionine)-modified carbon nanotube-coated carbon film electrode [44], magnetically entrapped SWCNT [45], uricase/carbon nanotube/carboxymethylcellulose electrode [46], UOx/AuNP/c-MWCNT/Au [47], and Naf/UOx/Fc/GCE [18]. The most sensitive biosensor among those analyzed was the zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase [43] with a limit of detection of 0.15 μM [43].
Table 3. Comparison of different CMEs for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| ZIF-11/GCE 1 | DP-ASV | 7.0 | UA, AA, G, sodium benzoate, saccharine, XA, hypoxanthine, KCl, Na2CO3, Na2SO4, CaCO3 | Human urine RR: 94.5–104.4% |
50–540 | 0.48 | [13] |
| NgB/CPE 2 | CV, DPV | 7.0 | UA, AA, DA | Human urine RR: 99.4–100.4% |
12.5–750 | 5 | [48] |
| ErGO/PEDOT:PSS/GCE 3 | DPV | - | UA, DA | Human urine RR: 96.8–109% |
10–100 | 1.08 | [49] |
| PMES/RGO/GCE 4 | CV | 7.0 | UA, AA, DA, L-Cys, L-Lys, L-Tyr, G | Human urine RR: 103.35% |
0.1–100 | 0.056 | [50] |
| NG/GCE 5 | DPV | 6.0 | UA, AA, DA | - | 0.1–20 | 0.045 | [51] |
| MC/GCE 6 | CV, DPV | 1.0 | UA, AA, DA | Synthetic urine RR: 101% |
10–150 | 1.7 | [52] |
| BDG-based electrode 7 | SWV | 2.25 | UA | Human urine RR: 95% RR: 95.2–103.1% |
8–1000 | 7.7 | [53] |
| PMB-ERGO/GCE 8 | SWV | 3.0 | UA, XA | Human urine RR: 97.8% |
0.08–400 | 0.03 | [14] |
| PEDOT-nf/PGE and Ox-PEDOT-nf/PGE 9 | CV | 2.0 | UA | Human urine, serum RR: 104–107% |
0.1–20 | 0.0013 | [41] |
| MWNTs/MGF/GCE 10 | DPV | 7.3 | UA, AA, DA, Trp, Na+, K+, Ca2+, Mg2+, G | - | 5–100; 300–10,000 | 0.93 | [9] |
| GCE/tosyl-CNPsE 11 | CV | 2.0 | UA, AA | Human urine RR: 106% |
0.1–100 | 0.2 | [42] |
| CTAB/GO/MWNTs/GCE 12 | DPV | 7.0 | UA, AA, DA, NO2− | Human urine RR: 99–115% |
3–600 | 1 | [40] |
| EGNWsE 13 | DPV | 7.4 | UA, AA, DA | - | 2.6–200 | 0.000033 | [54] |
| GEF/CFE 14 | DPV | 7.0 | UA, AA, DA | Human urine, serum | 3.98–371 | 2 | [55] |
| Trp-GR/GCE 15 | DPV | 7.0 | UA, AA, DA | Human urine RR: 97.3–99.9% Human serum RR: 92.6–98.7% |
10–1000 | 1.24 | [56] |
| NH2-VMSF/ErGO/SPCE 16 | DPV | 5.0 | UA, AA, DA, G, UR, Na+, K+, Ca2+, Mg2+ | Human whole blood RR: 99.0–107.0% |
0.5–180 | 0.129 | [57] |
AA = ascorbic acid, DA = dopamine, G = glucose, L-Cys = L-cystine, L-Lys = L-lysine, L-Tyr = L-tyrosine, SWV = square wave voltammetry, Trp = tryptophan, UA = uric acid, UR = urea, XA = xanthine; 1 = Zeolite Imidazolate Framework-11-modified electrode; 2 = Poly (Naphthol Green B)-film-modified carbon paste electrode; 3 = Gold nanoparticle-decorated polypyrrole/graphene oxide nanosheets; 4 = Poly(2-(N-morpholine)ethane sulfonic acid)/RGO-modified electrode; 5 = Nitrogen-doped graphene; 6 = Microporous carbon electrode; 7 = Boron-doped diamond electrode; 8 = Poly(Methylene Blue) and electrochemically reduced graphene oxide composite film-modified electrode; 9 = Over-oxidized poly(3,4-ethylenedioxythiophene) nanofiber-modified pencil graphite electrode; 10 = A multi-walled carbon nanotubes (MWNTs) bridged mesocellular graphene foam (MGF) nanocomposite (MWNTs/MGF)-modified glassy carbon electrode; 11 = Tosyl surface carbon nanoparticles/glassy carbon electrode; 12 = Hexadecyl trimethyl ammonium bromide (CTAB)-functionalized graphene oxide (GO)/multi-walled carbon nanotubes (MWNTs)-modified glassy carbon electrode; 13 = Three-dimensional (3D) unmodified ‘as-grown’ epitaxial graphene nanowall arrays (EGNWs); 14 = Carbon fiber electrode (CFE) modified by graphene flowers; 15 = Tryptophan-functionalized graphene nanocomposite (Trp-GR); 16 = Screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film.
Table 4. Comparison of biosensors for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) | UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| UOx/CNT/CMC 1 | CV | 7.4 | UA, AA, UR | Human urine, serum RR: 96.3% |
20–5000 | 2.8 | [46] |
| RGO/AuNP hybrid film 2 | Amperometry | 7.6 | UA, AA, DA | - | - | 1 | [58] |
| UOx-Th-SWNTs/GC 3 | - | - | UA, AA, 3,4-dihydroxyphenylacetic acid, 4-acetamidophenol | HEK 293A cells RR: 100.9–101.4% |
2–2000 | 0.5 | [59] |
| UOx/PBG/CNT/CFE and UOx PTH/CNT/CFE 4 | Amperometry | 7.0 | UA, AA, G, citric acid, creatinine, NH4+, phenol, UR | Human urine RR: 95–105% |
2–100 | 0.6 | [44] |
| UOx/rGO/ZnPc-NH2/GCE 5 | - | - | UA | Human urine RR: 92.5–97.6% |
0.5–100 | 0.15 | [43] |
| MP/SWCNT/SPE 6 | CV | 7.4 | UA, AA, DA | Human urine | 0.001–0.20 | 0.83 | [45] |
| UOx/AuNP/c-MWCNT/Au 7 | CV | 7.5 | UA, AA, G, chol, UR, pyruvate, bilirubin, CuSO4, KCl, FAD, NaCl, ZnSO4, NADH, CaCl2, EDTA, NEM, riboflavin, MnCl2, FM | Human serum RR: 95–97% |
5–800 | 5 | [47] |
| UOx- PANI-PB-PtE 8 | CV | 7.2 | UA, AA, UR, G | Human serum | 10–160 | 2.6 | [60] |
| UOx-PANI-MWCNT/ITO 9 | CV, DPV | - | UA | Human serum | 10–1000 | 10 | [61] |
| UOx/Nafion/ZnO-NFs/Au 10 | Amperometry | 7.4 | UA, AA, UR, G | - | 0.5–1500 | 0.5 | [62] |
| Naf/UOx/Fc/GCE 11 | DPV, Amperometry | 7.4 | UA, AA, DA, UR, G, XA | Human serum RR: 95% |
0.5–50; 25–600 |
0.23 | [63] |
AA = ascorbic acid, Chol = cholesterol, DA = dopamine, G = glucose, UA = uric acid, UR = urea. 1 = Uricase/carbon nanotube/carboxymethylcellulose electrode; 2 = Large-scale graphene film doped with gold nanoparticles; 3 = uricase − thionine − single-walled carbon nanotube-modified electrodes; 4 = Poly(brilliant green) and poly(thionine)-modified carbon nanotube-coated carbon film electrode; 5 = Zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase; 6 = Magnetically entrapped SWCNT; 7 = chitosan–glutaraldehyde crosslinked uricase immobilized onto Prussian blue nanoparticles (PBNPs) absorbed onto carboxylated multi-walled carbon nanotube (c-MWCNT) and polyaniline (PANI) layer, electrochemically deposited on the surface of Au electrode; 8 = Uricase-immobilized Polyaniline/Prussian blue (PANI-PB) composite on a platinum electrode (PtE); 9 = Uricase immobilized onto multi-walled carbon nanotubes (CNT) doped polyaniline (PANI) nanocomposite-indium tin oxide (ITO)-coated glass substrate; 10 = Uricase immobilized in conjunction with Nafion onto zinc oxide nanoflakes (ZnO-NF) and a gold-coated glass substrate; 11 = Ferrocene (Fc)-induced electro-activated uricase (UOx) deposited within Nafion (Naf) on glassy carbon electrode (GCE).
Considering future medical technologies, nanoparticles (NPs) will be central to developing new sensing devices because of their extremely small dimensions in the nanometer (nm) range, wide availability (found in nature or laboratory manufactured) and electrochemical properties [64][65].
The outlook for uric acid electroanalysis involves different configurations of nanocomposites. Carbon nanotubes (CNTs), including multi-walled nanotubes (MWCNTs) and single-walled nanotubes (SWCNTs) [66], are the most preferred for wide range of biological metabolites [67][68][69][70] because of their large specific surface area, enhanced activity and great stability. Moreover, nanocomposites amplify the electron transfer, resulting in a rapid and higher potential response [66].
The latest research is investigating sensors based on carbon nanotubes modified with lanthanum hydroxide (La(OH)3) [71], Zn_MM [72], cobalt phthalocyanine [66] or bentonite (Bent) and L-cysteine [73].
The scientific literature [74] also mentions different approaches and emerging strategies to develop reliable biosensors that consider different active and passive anti-biofouling strategies (Figure 2), thereby extending their applications to biological samples for clinical diagnostics, personalized medicine, point-of-care testing, and wearable devices.

Figure 2. Anti-biofouling strategies.
The abovementioned anti-biofouling strategies aim to delay the biofouling process and remove the accumulation of different bioactive compounds, if necessary. There is also the possibility of using combinations of different materials (materials with natural morphology and surface stability) and transducers to achieve a better long-term reliability.
References
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160.
- Chong, D.P. Theoretical Study of Uric Acid and its Ions in Aqueous Solution. J. Theor. Comput. Sci. 2013, 1.
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is it time to revise the normal range of serum uric acid levels? Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1295–1306.
- de Lassichere, C.; Latapie, L.; Evrard, D.; Gros, P. New Insight into the EC’ Mechanism of Uric Acid Regeneration in the Presence of Ascorbic Acid on a Poly(3,4-ethylenedioxithiophene) Modified Gold Electrode. Electroanalysis 2018, 30, 1653.
- Sajid, M.; Nazal, K.M.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. Trends Anal. Chem. 2016, 76, 15–29.
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.Y.; Del Campo, F.J. Development of amperometric biosensors based on nanostructured tyrosinase-conducting polymer, composite Electrodes. Sensors 2013, 13, 6759–6774.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162.
- Picillo, M.; Santangelo, G.; Moccia Erro, R.; Amboni, M.; Prestipino, E.; Longo, K.; Vitale, C.; Spina, E.; Orefice, G.; Barone, P.; et al. Serum uric acid is associated with apathy in early, drug naive Parkinson’s disease. J. Neural Transm. 2015, 2015, 371–377.
- Li, H.; Wang, Y.; Ye, D.; Luo, J.; Su, B.; Zhang, S.; Kong, J. An electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan based on MWNTs bridged mesocellular graphenefoam nanocomposite. Talanta 2014, 127, 255–261.
- Elhag, S.; Ibupoto, Z.H.; Nur, O.; Willander, M. Incorporating β-Cyclodextrin with ZnO nanorods: A potentiometric strategy for selectivity and detection of dopamine. Sensors 2014, 14, 1654–1664.
- Kumar, S.; Vicente-Beckett, V. Glassy carbon electrodes modified with multiwalled carbon nanotubes for the determination of ascorbic acid by square-wave voltammetry. Beilstein J. Nanotechnol. 2012, 3, 388–396.
- Levite, M. Dopamine and Tcells: Dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. 2016, 216, 42–89.
- Thanh, T.S.; Qui, P.T.; Thanh Tu, N.T.; Toan, T.T.T.; Hoa, T.T.B.; Son, L.V.T.; Nguyen, D.M.; Tuyen, T.N.; Khieu, D.Q. Electrochemical Determination of Uric Acid in Urine by Using Zeolite Imidazolate Framework-11 Modified Electrode. J. Nanomater. 2021, 2021, 9914062.
- Liu, G.; Wei, M.; Luo, Y.; Sun, D.; Shao, S. Simultaneous determination of uric acid and xanthine using a poly(methylene blue) and electrochemically reduced graphene oxide composite film modified electrode. J. Anal. Methods Chem. 2014, 2014, 984314.
- Guan, W.; Duan, X.; Reed, M.A. Highly specific and sensitive non-enzymatic determination of uric acid in serum and urine by extended gate field effect transistor sensors. Biosens. Bioelectron. 2014, 51, 225–231.
- Tadese, A.; Subramanian, P.A.; Woldu, A.; Pal, R. Electrochemical determination and comparison of ascorbic acid in freshly prepared and bottled fruit juices: A cyclic voltammetric study. J. Chem. Pharm. Res. 2014, 6, 880–888.
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418.
- Ide, K.; Yamada, H.; Umegaki, K.; Mizuno, K.; Kawakami, N.; Hagiwara, Y.; Matsumoto, M.; Yoshida, H.; Kim, K.; Shiosaki, E.; et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition 2015, 31, 406–408.
- Yue, H.Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Gunes, F.; Liu, L.C.; Lee, T.H.; Oh, E.S.; et al. ZnO Nanowire arrays on 3D hierachical graphene foam: Biomarker detection of Parkinson’s disease. ACS Chem. Neurosci. 2014, 8, 1639–1646.
- Sohouli, E.; Khosrowshahi, E.M.; Radi, P.; Naghian, E.; Rahimi-Nasrabadi, M.; Ahmadi, F. Electrochemical sensor based on modified methylcellulose by graphene oxide and Fe3O4 nanoparticles: Application in the analysis of uric acid content in urine. J. Electroanal. Chem. 2020, 877, 114503.
- Buledi, J.; Ameen, S.; Memon, S.; Fatima, A.; Solangi, A.; Mallah, A.; Karimi, F.; Malakmohammadi, S.; Agarwal, S.; Gupta, V. An Improved Non-Enzymatic Electrochemical Sensor Amplified with CuO Nanostructures for Sensitive Determination of Uric Acid. Open. Chem. 2021, 19, 481–491.
- Zare, H.R.; Ghanbari, Z.; Nasirizadeh, N.; Benvidi, A. Simultaneous Determination of Adrenaline, Uric Acid, and Cysteine Using Bifunctional Electrocatalyst of Ruthenium Oxide Nanoparticles. Comptes Rendus Chim. 2013, 3, 287–295.
- Rajeswari, B.; Reddy, K.V.N.S.; Devi, A.S.; Madhavi, G.; Reddy, I.R.V.S. Determination of Uric Acid Using TiO2 Nanoparticles Modified Glassy Carbon Electrode. Biointerface Res. Appl. Chem. 2022, 12, 6058–6065.
- Wei, Y.; Liu, Y.; Xu, Z.; Wang, S.; Chen, B.; Zhang, D.; Fang, Y. Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid Using a Novel Electrochemical Sensor Based on Palladium Nanoparticles/Reduced Graphene Oxide Nanocomposite. Int. J. Anal. Chem. 2020, 2020, 8812443.
- Sun, D.; Zhao, Q.; Tan, F.; Wang, X.; Gao, J. Simultaneous Detection of Dopamine, Uric Acid, and Ascorbic Acid Using SnO2 Nanoparticles/Multi-Walled Carbon Nanotubes/Carbon Paste Electrode. Anal. Methods 2012, 10, 3283.
- Wang, W.Q.; Yue, H.Y.; Yu, Z.M.; Huang, S.; Song, S.S.; Gao, X.; Guan, E.H.; Zhang, H.J.; Wang, Z. Synthesis and Application of MoS2 Nanosheet Arrays/Carbon Nanofibers for Simultaneous Electrochemical Determination of Levodopa and Uric Acid. IEEE Sens. J. 2019, 15, 5988–5994.
- Krishnamoorthy, K.; Sudha, V.; Kumar, S.M.S.; Thangamuthu, R. Simultaneous Determination of Dopamine and Uric Acid Using Copper Oxide Nano-Rice Modified Electrode. J. Alloys Compd. 2018, 748, 338–347.
- Fernandes, D.M.; Costa, M.; Pereira, C.; Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Novel electrochemical sensor based on N-doped carbon nanotubes and Fe3O4 nanoparticles: Simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. J. Colloid Interface Sci. 2014, 432, 207–213.
- Jiang, J.; Du, X. Sensitive Electrochemical Sensors for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid Based on Graphene Oxide Nanocomposites. Nanoscale 2014, 19, 11303–11309.
- Bai, Z.; Zhou, C.; Xu, H.; Wang, G.; Pang, H.; Ma, H. Polyoxometalates-Doped Au Nanoparticles and Reduced Graphene Oxide: A New Material for the Detection of Uric Acid in Urine. Sens. Actuators B Chem. 2017, 243, 361–371.
- Safitri, H.; Wahyuni, W.T.; Rohaeti, E.; Khalil, M.; Marken, F. Optimization of uric acid detection with Au nanorod-decorated graphene oxide (GO/AuNR) using response surface methodology. RSC Adv. 2022, 12, 25269–25278.
- Liu, K.; Zhang, J.; Yang, G.; Wang, C.; Zhu, J.-J. Direct Electrochemistry and Electrocatalysis of Hemoglobin Based on Poly(Diallyldimethylammonium Chloride) Functionalized Graphene Sheets/Room Temperature Ionic Liquid Composite Film. Electrochem. Commun. 2010, 3, 402–405.
- Imran, H.; Palinci, N.M.; Venkataraman, D. Facile and Green Synthesis of Graphene Oxide by Electrical Exfoliation of Pencil Graphite and Gold Nanoparticle for Non-Enzymatic Simultaneous Sensing of Ascorbic Acid, Dopamine and Uric Acid. RSC Adv. 2015, 78, 63513–63520.
- Filik, H.; Avan, A.A.; Aydar, S. Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Tryptophan with Azure A-Interlinked Multi-Walled Carbon Nanotube/Gold Nanoparticles Composite Modified Electrode. Arab. J. Chem. 2016, 3, 471–480.
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold Nanoparticle Decorated Polypyrrole/Graphene Oxide Nanosheets as a Modified Electrode for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. New. J. Chem. 2020, 12, 4916–4926.
- Abellán-Llobregat, A.; Vidal, L.; Rodríguez-Amaro, R.; Berenguer-Murcia, A.; Canals, A.; Morallón, E. Au-IDA Microelectrodes Modified with Au-Doped Graphene Oxide for the Simultaneous Determination of Uric Acid and Ascorbic Acid in Urine Samples. Electrochim. Acta 2017, 227, 275–284.
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A Facile Electrochemical Sensor Based on Reduced Graphene Oxide and Au Nanoplates Modified Glassy Carbon Electrode for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2014, 204, 302–309.
- Wang, S.; Zhang, W.; Zhong, X.; Chai, Y.; Yuan, R. Simultaneous Determination of Dopamine, Ascorbic Acid and Uric Acid Using a Multi-Walled Carbon Nanotube and Reduced Graphene Oxide Hybrid Functionalized by PAMAM and Au Nanoparticles. Anal. Methods 2015, 4, 1471–1477.
- Mazzara, F.; Patella, B.; Ganci, F.; O’Riordan, A.; Aiello, G.; Torino, C.; Vilasi, A.; Sunseri, C.; Inguanta, R. Flexible Electrode Based on Gold Nanoparticles and Reduced Graphene Oxide for Uric Acid Detection Using Linear Sweep Voltammetry. Chem. Eng. Trans. 2021, 87, 421–426.
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306.
- Ozcan, A.; Ilkbas, S. Preparation of poly(3,4-ethylenedioxythiophene) nanofibers modified pencil graphite electrode and investigation of over-oxidation conditions for the selective and sensitive determination of uric acid in body fluids. Anal. Chim. Acta 2015, 891, 312–320.
- Amiri, M.; Imanzadeh, H.; Banaei, A. Carbon nanoparticles with tosyl functional group for distinguishing voltammetric peaks of ascorbic acid and uric acid. Mater. Sci. Eng. 2015, C47, 189–195.
- Shi, Y.-M.; Mei, L.; Zhang, J.-X.; Hu, K.; Zhang, X.; Li, Z.-Z.; Miao, M.-S.; Li, X.-M. Synthesis of Zinc Tetraaminophthalocyanine Functionalized Graphene Nanosheets as an Enhanced Material for Sensitive Electrochemical Determination of Uric Acid. Electroanalysis 2020, 7, 1507–1515.
- Ghica, M.E.; Brett, C.M.A. Poly(brilliant green) and poly(thionine) modified carbon nanotube coated carbon film electrodes for glucose and uric acid biosensors. Talanta 2014, 130, 198–206.
- Herrastia, Z.; Martíneza, F.; Baldrich, E. Detection of uric acid at reversibly nanostructured thin-film microelectrodes. Sens. Actuators 2016, B234, 667–673.
- Fukuda, T.; Muguruma, H.; Iwasa, H.; Tanaka, T.; Hiratsuka, A.; Shimizu, T.; Tsuji, K.; Kishimoto, T. Electrochemical Determination of Uric Acid in Urine and Serum with Uricase/Carbon Nanotube/Carboxymethylcellulose Electrode. Anal. Biochem. 2020, 590, 113533.
- Rawal, R.; Chawla, S.; Chauhan, N.; Dahiya, T.; Pundir, C.S. Construction of amperometric uric acid biosensor based on uricase immobilized on PBNPs/cMWCNT/PANI/Au composite. Int. J. Biol. Macromol. 2012, 50, 112–118.
- Chitravathi, S.; Kumara Swamy, B.E.; Mamatha, G.P.; Sherigara, B.S. Electrochemical Behavior of Poly (Naphthol Green B)-Film Modified Carbon Paste Electrode and Its Application for the Determination of Dopamine and Uric Acid. J. Electroanal. Chem. 2012, 667, 66–75.
- Gao, N.; Yu, J.; Tian, Q.; Shi, J.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and Its Composites in Electrochemical and Electronic Chemosensors. Chemosensors 2021, 9, 79.
- Zhang, K.; Zhang, N.; Zhang, L.; Wang, H.; Shi, H.; Liu, Q. Simultaneous voltammetric detection of dopamine, ascorbic acid and uric acid using a poly(2-(N-morpholine)ethane sulfonic acid)/RGO modified electrode. RSC Adv. 2018, 8, 5280–5285.
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131.
- Rattanaumpa, T.; Maensiri, S.; Ngamchuea, K. Microporous carbon in the selective electro-oxidation of molecular biomarkers: Uric acid, ascorbic acid, and dopamine. RSC Adv. 2022, 12, 18709–18721.
- Cinkova, K.; Kianičkova, K.; Stanković, D.M.; Vojs, M.; Marton, M.; Švorc, Ľ. The doping level of boron-doped diamond electrodes affects the voltammetric sensing of uric acid. Anal. Methods 2018, 10, 991–996.
- Roy, P.K.; Ganguly, A.; Yang, W.K.; Wu, C.T.; Hwang, J.S.; Tai, Y.; Chen, K.H.; Chen, L.C.; Chattopadhyay, S. Edge promoted ultrasensitive electrochemical detection of organic bio-molecules on epitaxial graphene nanowalls. Biosens. Bioelectron. 2015, 70, 137–144.
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novelgraphene flowersmodified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224.
- Lian, Q.; He, Z.; He, Q.; Luo, A.; Yan, K.; Zhang, D.; Lu, X.; Zhou, X. Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal. Chim. Acta 2014, 823, 32–39.
- Sayyad, P.W.; Sontakke, K.S.; Farooqui, A.A.; Shirsat, S.M.; Tsai, M.-L.; ShirsaT, M.D. A Novel Three-Dimensional Electrochemical Cd(II) Biosensor Based on l-Glutathione Capped Poly(3,4-Ethylenedioxythiophene):Polystyrene Sulfonate/Carboxylated Multiwall CNT Network. J. Sci. Adv. Mater. Devices 2022, 7, 100504.
- Zhang, P.; Huang, Y.; Lu, X.; Zhang, S.; Li, J.; Wei, G.; Su, Z. One-Step Synthesis of Large-Scale Graphene Film Doped with Gold Nanoparticles at Liquid–Air Interface for Electrochemistry and Raman Detection Applications. Langmuir. 2014, 30, 8980–8989.
- Chen, D.; Wang, Q.; Jin, J.; Wu, P.; Wang, H.; Yu, S.; Zhang, H.; Cai, C. Low-potential detection of endogenous and physiological uric acid at uricase − thionine − single-walled carbon nanotube modified electrodes. Anal. Chem. 2010, 82, 2448–2455.
- Thakur, B.; Sawant, S.N. Polyaniline/Prussian-Blue-Based Amperometric Biosensor for Detection of Uric Acid. ChemPlusChem 2012, 2, 166–174.
- Arora, K.; Choudhary, M.; Malhotra, B.D. Enhancing performance of uricase using multiwalled carbon nanotube doped polyaniline. Appl. Biochem. Biotechnol. 2014, 174, 1174–1187.
- Usman, A.S.M.; Ibupoto, Z.H.; Kashif, M.; Hashim, U.; Willander, M. A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase. Sensors 2012, 12, 2787–2797.
- Ghoh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase, electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9.
- Hwang, C.; Lee, W.J.; Kim, S.D.; Park, S.; Kim, J.H. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors 2022, 12, 1020.
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors 2023, 23, 2240.
- Balogun, S.A.; Fayemi, O.E. Recent Advances in the Use of CoPc-MWCNTs Nanocomposites as Electrochemical Sensing Materials. Biosensors 2022, 12, 850.
- Li, Q.; Wang, Z.; Liang, Q.; Zhou, M.; Xu, S.; Li, Z.; Sun, D. Tetra-substituted cobalt (II) phthalocyanine/multi-walled carbon nanotubes as new efficient catalyst for the selective oxidation of styrene using tert-butyl hydroperoxide. Fuller. Nanotub. Carbon. Nanostruct. 2020, 28, 799–807.
- Şavk, A.; Özdil, B.; Demirkan, B.; Nas, M.S.; Calimli, M.H.; Alma, M.H.; Asiri, A.M.; Şen, F. Multiwalled carbon nanotube-based nanosensor for ultrasensitive detection of uric acid, dopamine, and ascorbic acid. Mater. Sci. Eng. C 2019, 99, 248–254.
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L.J. Ferrocene Functionalized Gold Nanoparticles on Carbon Nanotube Electrodes for Portable Dopamine Sensor. In Proceedings of the Electrochemical Society Meeting Abstracts 239, Digital Meeting, 30 May–3 June 2021; Volume 55, p. 1345.
- Musarraf, H.M.; Asiri, A.M.; Uddin, J.; Marwani, H.M.; Rahman, M.M. Development of a L-cysteine Sensor Based on Thallium Oxide Coupled Multi-walled Carbon Nanotube Nanocomposites with Electrochemical Approach. Chem. Asian J. 2022, 17, e202101117.
- Knežević, S.; Ognjanović, M.; Stanković, V.; Zlatanova, M.; Nešić, A.; Gavrović-Jankulović, M.; Stanković, D. La(OH)3 Multi-Walled Carbon Nanotube/Carbon Paste-Based Sensing Approach for the Detection of Uric Acid—A Product of Environmentally Stressed Cells. Biosensors 2022, 12, 705.
- Negrea, S.; Andelescu, A.A.; Ilies, B.; Motoc, S.; Cretu, C.; Cseh, L.; Rastei, M.; Donnio, B.; Szerb, E.I.; Manea, F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomater. 2022, 12, 4215.
- Choukairi, M.; Bouchta, D.; Bounab, L.; González-Romero, E.; Achache, M.; Draoui, K.; Chaouket, F.; Raissouni, I.; Gharous, M. A Carbon Paste Electrode Modified by Bentonite and l-Cysteine for Simultaneous Determination of Ascorbic and Uric Acids: Application in Biological Fluids. ChemistryOpen 2023, 12, e202200201.
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66.
More
Information
Subjects:
Medical Laboratory Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
25 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




