Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Mihaela Badea and Version 3 by Catherine Yang.
Uric acid is a metabolic product that results from degradation of purines in the liver. Usually, uric acid is identified from biological fluids, human serum and urine through conventional methods, such as spectroscopy, chromatography, electrochemistry, membrane capillary electrophoresis and spectrophotometric methods, including uricase enzymatic reactions. Importantly, uric acid determination opens the possibility of early intervention in cases of hyperuricemia and preventing the degradation of renal function.
- uric acid
- chemosensors
- biosensors
- nanocomposites
1. Introduction
From the electrochemical point of view, uric acid is a weak acid, with two-step dissociation at a pKa1 of 5.4 and a pKa2 of 9.8. In the physiological range of pH (7.35–7.45), in the extracellular compartment, uric acid is found mostly (98%) in the form of biurate (deprotonated urate anion or ionized urate), and a very small quantity (<1%) is found as undissociated uric acid [1][2]. However, in more acid pH media, such as urine (pH 6.5), uric acid is still found mainly as biurate (88%) but with an increased percentage as uric acid (12%) [1][2][2,3].
The physiological levels of uric acid are between 3.5 mg/dL and 7.2 mg/dL (210 μM and 430 μM) in males, and between 2.6 mg/dL and 6.0 mg/dL (155 μM and 360 μM) in premenopausal females [1][2]. These levels are maintained by exogenous input (diet) but mostly by endogenous formation (nucleic acid catabolism and de novo synthesis) [3][4].
At high levels of uric acid, hyperuricemia, the undissociated uric acid precipitates at the vascular level and biurate is implicated in kidney stones formation. This phenomenon occurs because of the low solubility (6 mg/dL or 360 µM) of uric acid, mainly in the form of monosodium urate [1][2].
The oxidation of uric acid starts with the formation of diimine (a) by exchanging 2e− and 2H+. The resulting diimine takes up two molecules of water and forms imine-alcohol (b) and uric acid-4,5-diol (c), successively. Ultimately, uric acid-4,5-diol is decomposed to allantoin (d) and CO2 in neutral pH (Figure 1) [4][5].
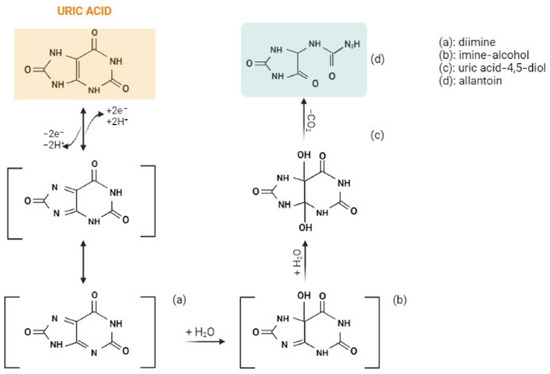
Figure 1.
The electrochemical oxidation of uric acid to allantoin. Created with BioRender.com.
The oxidative properties of uric acid can be used in developing catalytic methods of detection. Thanks to the high electrochemical capacity of uric acid, for the rapid quantification of uric acid levels, scientists have developed different uric acid detection tools.
Together with uric acid, dopamine and ascorbic acid have similar oxidative behavior and coexist in urine samples [5][6]. Therefore, uric acid, dopamine and ascorbic acid signals can interfere with each other in the process of electrochemical detection in real samples. These three compounds have a very similar oxidation potential, so their electrochemical detection is very challenging [5][6] as obtaining separate voltametric peaks is the principal objective [6][7]. This matter has been investigated frequently for most types of electrodes, such as conventional sensors, modifiable electrodes and biosensors.
However, dopamine, uric acid and ascorbic acid have individual and cumulative importance because of their role in oxidative stress-related diseases [7][8]. Parkinson’s disease, most of all, lacks a rapid diagnostic method using biological markers for diagnosis of the early stages of the pathology [8][9] and it is an example where simultaneous detection of the three compounds may be useful [9][10].
Other cases in which it may be important to establish levels of uric acid and its electrochemically similar compounds, dopamine and ascorbic acid, in biological matrixes are the following: dopamine: cardiotoxicity [10][11], aging [11][12], multiple sclerosis [12][13], rheumatoid arthritis [12][13], Alzheimer’s disease [12][13], and Tourette [12][13]; uric acid: arthritis [13][14], gout [13][14], Lesch–Nyhan syndrome [13][14], urolithiasis [13][14], kidney damage [13][14], leukemia [14][15], lymphoma [14][15], and multiple sclerosis [15][16]; and ascorbic acid: high blood pressure [16][17], heart attack risk [16][17], cataracts [16][17], tooth decay [16][17], improper bone development [16][17], loss of appetite [16][17], weakened cartilage [16][17], skin hemorrhages [16][17], impaired digestion [16][17], septic shock [17][18], and diabetes mellitus [18][19].
24. Uric Acid Electrochemical Detection
Among the transition metal oxide-modified electrodes, ZnO NWAs/GF/GCE [19][24] had the best performance in terms of sensitivity, but highly selective sensors with moderately higher limits of detection included GCE/MC–GO–Fe3O4 [20][37], CuO/GCE [21][40] and RuON-GCE [22][51] (Table 1).Table 1.
Comparison of electrodes modified with transition metal nanoparticles for detection of uric acid.
| UA LOD (μM) | Ref. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human urine | RR: 94.5–104.4% | 50–540 | 0.48 | [ | 13 | ] | [14] | |||||||||||
| UOx/CNT/CMC | 1 | CV | 7.4 | UA, AA, UR | Human urine, serum RR: 96.3% |
20–5000 | 2.8 | [46] | [101] | |||||||||
| NgB/CPE | 2 | CV, DPV | 7.0 | UA, AA, DA | Human urine RR: 99.4–100.4% |
12.5–750 | 5 | |||||||||||
| RGO/AuNP hybrid film | 2 | [ | 48 | ] | [ | 74 | ] | |||||||||||
| Amperometry | 7.6 | UA, AA, DA | - | - | 1 | [ | 58] | [55] | ErGO/PEDOT:PSS/GCE | 3 | DPV | - | UA, DA | Human urine RR: 96.8–109% |
10–100 | 1.08 | [49] | |
| UOx-Th-SWNTs/GC | 3 | [ | - | 76 | - | ] | ||||||||||||
| UA, AA, 3,4-dihydroxyphenylacetic acid, 4-acetamidophenol | HEK 293A cells | RR: 100.9–101.4% | 2–2000 | 0.5 | [ | 59] | [96] | PMES/RGO/GCE | 4 | CV | 7.0 | UA, AA, DA, L-Cys, L-Lys, L-Tyr, G | Human urine RR: 103.35% |
0.1–100 | 0.056 | [50] | [75] | |
| UOx/PBG/CNT/CFE and UOx PTH/CNT/CFE | 4 | Amperometry | 7.0 | UA, AA, G, citric acid, creatinine, NH | 4+ | , phenol, UR | Human urine RR: 95–105% |
2–100 | 0.6 | [44] | [97 | |||||||
| - | 25–85 | 0.47 | [ | 28 | ] | [ | 22] | |||||||||||
| ZnO NWAs/GF/GCE | 10 | DPV | 7.4 | UA, AA, DA | Human serum | 0–40 | 0.001 | [19] | [24] |
AA = ascorbic acid, Cys = cysteine, DA = dopamine, E = epinephrine, G = glucose, SWV = square wave voltammetry, UA = uric acid, UR = urea; 1 = Glassy carbon electrode based on modified methylcellulose by graphene oxide and Fe3O4 nanoparticles; 2 = Glassy carbon electrode coated with titanium dioxide nanoparticles; 3 = Palladium nanoparticles/reduced graphite oxide nanocomposite on a glassy carbon electrode; 4 = SnO2 nanoparticles/multi-walled carbon nanotubes/carbon paste electrode; 5 = Glassy carbon electrode coated with copper oxide; 6 = Glassy carbon electrode modified with ruthenium oxide nanoparticles; 7 = MoS2 nanosheet arrays/carbon nanofibers; 8 = CuO nano-rice-modified glassy carbon electrode; 9 = N-doped carbon nanotubes functionalized with Fe3O4 nanoparticles; 10 = Glassy carbon electrode modified with ZnO nanowire arrays on 3D graphene foam.
Table 2.
Comparison of gold-coated electrodes for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GO/AuNR/GCE | 1 | DPV | - | UA, AA, DA, G, UR, Mg | 2+ | Human urine | 10–90 | 0.4 | [31] | [27] | ||||||||||||||||||||||||
| AuNPs@GO/PPy/CFP | 2 | DPV | 7.0 | UA, AA, DA | Human urine RR: 96.8–109% |
2–360 | 1.68 | [35] | [30] | |||||||||||||||||||||||||
| AuNPs-GO/Au-IDA | 3 | CV | 7.0 | UA, AA, DA, G, E | Human urine | 2–1050 | 0.62 | [36] | [28] | |||||||||||||||||||||||||
| GCE-PErGO-AuNP | 4 | CV, DPV | 7.4 | UA, AA, DA | Human urine | 20–260 | 20 | [33] | [31] | |||||||||||||||||||||||||
| ] | AuRGO/GCE | 5 | DPV | 7.0 | NG/GCE UA, AA, DA | 5 | Human serum | DPV | 6.0 | UA, AA, DA | - | 0.1–20 | 0.045 | |||||||||||||||||||||
| UOx/rGO/ZnPc-NH | 2 | /GCE | 5 | - | - | [ | UA RR: 97.5–102% |
88–53 | 1.8 | [37] | [32] | |||||||||||||||||||||||
| Human urine | RR: 92.5–97.6% | 51 | ] | [ | 0.5–100 | 87] | ||||||||||||||||||||||||||||
| 0.15 | [ | 43 | ] | Au@Pd-RGO/GCE | 6 | DPV | MC/GCE 7.0 | 6 | UA, AA, DA | Human urine RR: 97.1–102.5% |
0.02–500; 0.1–350 |
0.005; 0.02 |
[29] | [33] | ||||||||||||||||||||
| CV, DPV | 1.0 | UA, AA, DA | Synthetic urine | RR: 101% |
10–150 | 1.7 | [52] | [88] | ||||||||||||||||||||||||||
| [ | 103 | ] | 98 | ] | PEI/[P | 2 | W | 16 | V | 2 | -Au/PDDA-rGO] | 8 | 7 | BDG-based electrode DPV | 7 | 7.0 | UA, AA, DA, NaCl, KCl, NH | 4 | Cl, L-Cys, L-Glu, CA, UR, G | Human urine RR: 95.2–103.1% |
||||||||||||||
| UOx/AuNP/c-MWCNT/Au | 0.25–1500 | 0.08 | SWV | 7 | 2.25 | [ | CVUA | 7.5 | UA, AA, G, chol, UR, pyruvate, bilirubin, CuSO | 4 | , KCl, FAD, NaCl, ZnSO | 4 | , NADH, CaCl | 2 | , EDTA, NEM, riboflavin, MnCl | 2 | , FMHuman urine RR: 95% RR: 95.2–103.1% | 30 | Human serum8–1000 | 7.7 |
RR: 95–97% | [ | 5–800 | ] | [34] | |||||||||
| 53 | ] | [ | 5 | 89 | ] | rGO-PAMAM-CNT-Au | 8 | |||||||||||||||||||||||||||
| [ | 47 | ] | [ | 102 | ] | DPV | 4.0 | UA, AA, DA | - | 1–114 | 0.33 | PMB-ERGO/GCE | 8 | SWV | 3.0 | UA, XA | Human urine RR: 97.8% | [38] | ||||||||||||||||
| UOx- PANI-PB-PtE | 8 | [ | 35 | CV | ] | |||||||||||||||||||||||||||||
| 7.2 | UA, AA, UR, G | 0.08–400 | 0.03 | [ | 14 | ] | [15] | |||||||||||||||||||||||||||
| Human serum | 10–160 | 2.6 | [ | 60 | ] | [ | 99] | Naf/AuNPs/AzA/MWCNTs | 9 | PEDOT-nf/PGE and Ox-PEDOT-nf/PGE DPV | 9 | 7.0 | UA, AA, DA, Trp, Na | + | , K | + | , Ca | 2+ | , Mg | 2+ | , G, citric acid, tartaric acid | Human urine RR: 99.7–103% |
0.5–50 | 0.28 | CV | [34] | [36] | |||||||
| 2.0 | UA | |||||||||||||||||||||||||||||||||
| UOx-PANI-MWCNT/ITO | 9 | CV, DPV | Human urine, serum | RR: 104–107% |
0.1–20 | 0.0013 | [41 | - | UA | Human serum | 10–1000 | ] | [90 | 10] | ||||||||||||||||||||
| [ | 61 | ] | [ | 100 | ] | ITO-rGO-AuNPs | 10 | MWNTs/MGF/GCE | 10 | LSV | 8.0 | UA, AA, Cl, Na | + | , Ca | 2+ | NH | 4+ | DPV | 7.3 | UA, AA, DA, Trp, NaHuman urine, milk | 10–500 | 3.6 | + | , K | + | , Ca | 2+ | , Mg | 2+ | , G | [ | - | 39] | [65] |
| 5–100; 300–10,000 | 0.93 | [ | ||||||||||||||||||||||||||||||||
| UOx/Nafion/ZnO-NFs/Au | 10 | Amperometry | 9 | ] | [ | 10 | ] | |||||||||||||||||||||||||||
| 7.4 | UA, AA, UR, G | GCE/tosyl-CNPsE | 11 | CV | 2.0 | UA, AA | Human urine RR: 106% |
0.1–100 | 0.2 | [42] | [91] | |||||||||||||||||||||||
| CTAB/GO/MWNTs/GCE | 12 | DPV | 7.0 | UA, AA, DA, NO | 2− | Human urine RR: 99–115% |
- | 2.6–200 | 0.000033 | [54] | [93] | |||||||||||||||||||||||
| GEF/CFE | 14 | DPV | ||||||||||||||||||||||||||||||||
| MP/SWCNT/SPE | 6 | CV | 7.4 | UA, AA, DA | Human urine | 0.001–0.20 | 0.83 | [45] | [ | - | 0.5–1500 | 0.5 | [62] | [104] | EGFET-AuE | 11 | - | 7.0 | UA, AA, G, bilirubin, hemoglobin | Human urine, serum | ||||||||||||||
| Naf/UOx/Fc/GCE | 11 | DPV, Amperometry | 1–1000 | 0.5 | [ | 15 | 7.4 | ] | [16] | |||||||||||||||||||||||||
| UA, AA, DA, UR, G, XA | ||||||||||||||||||||||||||||||||||
| Human serum | ||||||||||||||||||||||||||||||||||
| RR: 95% | ||||||||||||||||||||||||||||||||||
| 0.5–50; | ||||||||||||||||||||||||||||||||||
| 25–600 | ||||||||||||||||||||||||||||||||||
| 0.23 | [ | 63 | ] | [ | 23 | ] | ||||||||||||||||||||||||||||
| 3–600 | 1 | [ | 40 | ] | [ | 92 | ] | |||||||||||||||||||||||||||
| EGNWsE | 13 | DPV | 7.4 | UA, AA, DA | ||||||||||||||||||||||||||||||
| 7.0 | UA, AA, DA | Human urine, serum | 3.98–371 | 2 | [ | 55 | ] | [94] | ||||||||||||||||||||||||||
| Trp-GR/GCE | 15 | DPV | 7.0 | UA, AA, DA | Human urine RR: 97.3–99.9% Human serum RR: 92.6–98.7% |
10–1000 | 1.24 | [56] | [95] | |||||||||||||||||||||||||
| NH | 2 | -VMSF/ErGO/SPCE | 16 | DPV | 5.0 | UA, AA, DA, G, UR, Na | + | , K | + | , Ca | 2+ | , Mg | 2+ | Human whole blood RR: 99.0–107.0% |
0.5–180 | 0.129 | [57] | [86] | ||||||||||||||||
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. | |||||||||||||||||||||||||||
| GCE/MC–GO–Fe | 3 | O | 4 | 1 | CV, DPV | 7.0 | UA, AA, DA, G, sucrose, L-Cys, citric acid, Fe | 2+ | , Cl | − | , Na | + | , NO | 3− | Human urine RR > 96% |
0.5–140 | 0.17 | [20] | [37] | |||||||||||||||
| TiO | 2 | NPs/GCE | 2 | DPV | 7.0 | UA | Human urine RR: 97–99.6% |
1–9 | 0.764 | [23] | [39] | |||||||||||||||||||||||
| PdNPs/rGO/GCE | 3 | DPV | 7.2 | UA, AA, DA | Human serum RR: 96.6–108.5% |
0.3–1400 | 16.67 | [24] | [42] | |||||||||||||||||||||||||
| SnO | 2 | /chitosan/GCE | 4 | DPV | UA, AA, DA | Human urine RR: 97.4% |
3–200 | 1 | [25] | [50] | ||||||||||||||||||||||||
| CuO/GCE | 5 | CV | 7.4 | UA, UR, lactic acid, ethanol, G, K | + | , Na | + | Human urine RR: 95–104% |
0.001–351,000 | 0.6 | [21] | [40] | ||||||||||||||||||||||
| RuON-GCE | 6 | DPV | 7.0 | UA, E | Human urine RR: 98–101.6% |
3.0–56.6; 56.6–758.6 | 0.47 | [22] | [51] | |||||||||||||||||||||||||
| MoS | 2 | NSA/CNFs | 7 | CV, DPV | 7.0 | UA, levodopa | Human urine RR: 99.7–102.6% |
1–60 | 1 | [26] | [52] | |||||||||||||||||||||||
| CuO nano-rice/GCE | 8 | CV, DPV | 7.0 | UA, AA, DA, G, fructose, galactose, lactose, Na | + | , Cl | − | , K | + | , Ca | 2+ | , Br | − | , CO | 23− | , NH4 | + | , NO | 2− | , NO | 3− | , SO | 42− | , SO | 32− | Human urine RR: 98.6–102.6% |
1–60 | 1.2 | [27] | [53] | ||||
| Fe | 3 | O | 4 | @CNT-N/GCE | 9 | SWV | 2.5 | UA, AA, DA |
AA = ascorbic acid, CA = citric acid, Cys = cysteine, DA = dopamine, E = epinephrine, G = glucose, Glu = glutamate, LSV = linear sweep voltammetry, Trp = tryptophan, UA = uric acid, UR = urea. 1 = Gold nanorod-decorated graphene oxide glassy carbon electrode; 2 = Gold nanoparticles-decorated polypyrole/graphene oxide nanosheets; 3 = Gold interdigitated microelectrodes array modified with graphene oxide and doped with gold nanoparticles; 4 = Gold nanoparticles deposition on reduced graphene oxide based on glassy carbon electrode; 5 = Reduced graphene oxide and gold nanoplates-modified glassy carbon electrode; 6 = Reduced graphene oxide-supported bimetallic, gold-palladium nanocomposites; 7 = Poly(diallyldimethylammonium chloride)-functionalized reduced graphene oxide and polyoxometalates-doped gold nanoparticles sensor; 8 = sensor based on reduced graphene oxide functionalized by poly(amido-amine), multi-walled carbon nanotubes and gold nanoparticles; 9 = Nafion-based electrode modified with Azure A-coated carbon nanotubes coated with gold nanoparticles; 10 = polyethylene terephthalate substrate coated with indium tin oxide and combined with reduced graphene oxide and gold nanoparticles; 11 = 11-(ferrocenyl)undecanethiol-modified gold electrode.
Regarding the chemosensors, most of the sensors are highly sensitive for detection of uric acid (Table 3). Some sensors demonstrated good sensitivity and higher specificity, including ZIF-11/GCE [13][14], boron-doped diamond electrode [40][92], over-oxidized poly(3,4-ethylenedioxythiophene) nanofibers/PGE [41][90], and tosyl surface carbon nanoparticles/GCE [42][91]. Table 4 shows a large variety of biosensors with good and high selectivity for uric acid from real samples: zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase [43][103], poly(brilliant green) and poly(thionine)-modified carbon nanotube-coated carbon film electrode [44][97], magnetically entrapped SWCNT [45][98], uricase/carbon nanotube/carboxymethylcellulose electrode [46][101], UOx/AuNP/c-MWCNT/Au [47][102], and Naf/UOx/Fc/GCE [18][19]. The most sensitive biosensor among those analyzed was the zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase [43][103] with a limit of detection of 0.15 μM [43][103].
Table 3.
Comparison of different CMEs for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) |
UA Linear Range (μM) | UA LOD (μM) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIF-11/GCE | 1 | DP-ASV | 7.0 | UA, AA, G, sodium benzoate, saccharine, XA, hypoxanthine, KCl, Na | 2 | CO | 3 | , Na | 2 | SO | 4 | , CaCO | 3 |
AA = ascorbic acid, DA = dopamine, G = glucose, L-Cys = L-cystine, L-Lys = L-lysine, L-Tyr = L-tyrosine, SWV = square wave voltammetry, Trp = tryptophan, UA = uric acid, UR = urea, XA = xanthine; 1 = Zeolite Imidazolate Framework-11-modified electrode; 2 = Poly (Naphthol Green B)-film-modified carbon paste electrode; 3 = Gold nanoparticle-decorated polypyrrole/graphene oxide nanosheets; 4 = Poly(2-(N-morpholine)ethane sulfonic acid)/RGO-modified electrode; 5 = Nitrogen-doped graphene; 6 = Microporous carbon electrode; 7 = Boron-doped diamond electrode; 8 = Poly(Methylene Blue) and electrochemically reduced graphene oxide composite film-modified electrode; 9 = Over-oxidized poly(3,4-ethylenedioxythiophene) nanofiber-modified pencil graphite electrode; 10 = A multi-walled carbon nanotubes (MWNTs) bridged mesocellular graphene foam (MGF) nanocomposite (MWNTs/MGF)-modified glassy carbon electrode; 11 = Tosyl surface carbon nanoparticles/glassy carbon electrode; 12 = Hexadecyl trimethyl ammonium bromide (CTAB)-functionalized graphene oxide (GO)/multi-walled carbon nanotubes (MWNTs)-modified glassy carbon electrode; 13 = Three-dimensional (3D) unmodified ‘as-grown’ epitaxial graphene nanowall arrays (EGNWs); 14 = Carbon fiber electrode (CFE) modified by graphene flowers; 15 = Tryptophan-functionalized graphene nanocomposite (Trp-GR); 16 = Screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film.
Table 4. Comparison of biosensors for detection of uric acid.
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) | UA Linear Range (μM) |
|---|
AA = ascorbic acid, Chol = cholesterol, DA = dopamine, G = glucose, UA = uric acid, UR = urea. 1 = Uricase/carbon nanotube/carboxymethylcellulose electrode; 2 = Large-scale graphene film doped with gold nanoparticles; 3 = uricase − thionine − single-walled carbon nanotube-modified electrodes; 4 = Poly(brilliant green) and poly(thionine)-modified carbon nanotube-coated carbon film electrode; 5 = Zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase; 6 = Magnetically entrapped SWCNT; 7 = chitosan–glutaraldehyde crosslinked uricase immobilized onto Prussian blue nanoparticles (PBNPs) absorbed onto carboxylated multi-walled carbon nanotube (c-MWCNT) and polyaniline (PANI) layer, electrochemically deposited on the surface of Au electrode; 8 = Uricase-immobilized Polyaniline/Prussian blue (PANI-PB) composite on a platinum electrode (PtE); 9 = Uricase immobilized onto multi-walled carbon nanotubes (CNT) doped polyaniline (PANI) nanocomposite-indium tin oxide (ITO)-coated glass substrate; 10 = Uricase immobilized in conjunction with Nafion onto zinc oxide nanoflakes (ZnO-NF) and a gold-coated glass substrate; 11 = Ferrocene (Fc)-induced electro-activated uricase (UOx) deposited within Nafion (Naf) on glassy carbon electrode (GCE).
Considering future medical technologies, nanoparticles (NPs) will be central to developing new sensing devices because of their extremely small dimensions in the nanometer (nm) range, wide availability (found in nature or laboratory manufactured) and electrochemical properties [64][65][105,106].
The outlook for uric acid electroanalysis involves different configurations of nanocomposites. Carbon nanotubes (CNTs), including multi-walled nanotubes (MWCNTs) and single-walled nanotubes (SWCNTs) [66][107], are the most preferred for wide range of biological metabolites [67][68][69][70][108,109,110,111] because of their large specific surface area, enhanced activity and great stability. Moreover, nanocomposites amplify the electron transfer, resulting in a rapid and higher potential response [66][107].
The latest research is investigating sensors based on carbon nanotubes modified with lanthanum hydroxide (La(OH)3) [71][112], Zn_MM [72][113], cobalt phthalocyanine [66][107] or bentonite (Bent) and L-cysteine [73][114].
The scientific literature [74][115] also mentions different approaches and emerging strategies to develop reliable biosensors that consider different active and passive anti-biofouling strategies (Figure 23), thereby extending their applications to biological samples for clinical diagnostics, personalized medicine, point-of-care testing, and wearable devices.
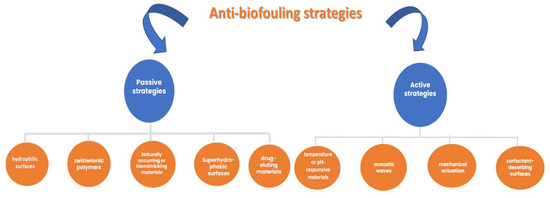 The abovementioned anti-biofouling strategies aim to delay the biofouling process and remove the accumulation of different bioactive compounds, if necessary. There is also the possibility of using combinations of different materials (materials with natural morphology and surface stability) and transducers to achieve a better long-term reliability.
The abovementioned anti-biofouling strategies aim to delay the biofouling process and remove the accumulation of different bioactive compounds, if necessary. There is also the possibility of using combinations of different materials (materials with natural morphology and surface stability) and transducers to achieve a better long-term reliability.

Figure 23.
Anti-biofouling strategies.
