
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tomás Cerdó | -- | 1165 | 2023-06-20 13:45:35 | | | |
| 2 | Wendy Huang | Meta information modification | 1165 | 2023-06-21 05:57:29 | | |
Video Upload Options
Functional gastrointestinal diseases (FGIDs) are characterized by recurring gastrointestinal symptoms, including abdominal pain, vomiting or constipation, that can ultimately cause non-optimal development, disrupt digestion or create lifelong or mortal complications. Scientific evidence suggests that both gastrointestinal diseases (GIDs) and FGIDs are related to gut microbial dysbiosis, gastrointestinal motility disturbance, visceral hypersensitivity, as well as impairment of mucosal immune function and central nervous system processing. Based on this, the use of probiotic bacteria, mainly those strains belonging to Lactobacillus, Bifidobacterium and probiotic yeast such as Saccharomyces, have emerged as potential therapeutic agents in GIDs.
1. Introduction
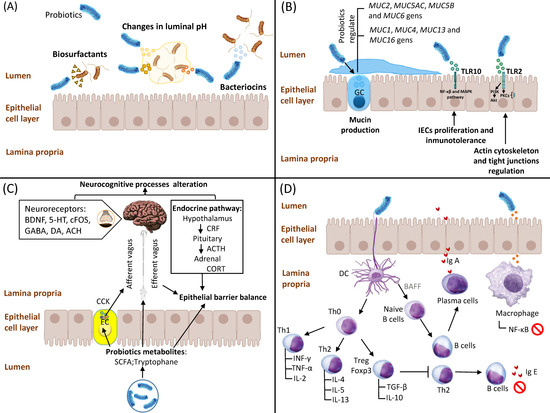
2. Competitive Adhesion and Exclusion of Potential Pathogens
3. Stimulation of IEC Proliferation and Epithelial Barrier Enhancement
4. Interaction of Probiotics with the Enteric Nervous System
5. Interaction of Probiotics with the Immune System
References
- Azad, A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630.
- Rolfe, R.D. Population dynamics of the intestinal tract. In Colonization Control of Human Bacterial Enteropathologens in Poultry; Academic Press: Cambridge, MA, USA, 1991; Volume 59.
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.-M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tomé, D. Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences. Am. J. Pathol. 2017, 187, 476–486.
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Novel formulations for antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 18040–18083.
- Hajfarajollah, H.; Eslami, P.; Mokhtarani, B.; Akbari, N.K. Biosurfactants from probiotic bacteria: A review. Biotechnol. Appl. Biochem. 2018, 65, 768–783.
- Rodrigues, L.R.; Teixeira, J.A.; van der Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf. B. Biointerfaces 2006, 49, 79–86.
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639.
- Goto, Y. Epithelial Cells as a Transmitter of Signals from Commensal Bacteria and Host Immune Cells. Front. Immunol. 2019, 10, 2057.
- Petersson, J.; Schreiber, O.; Hansson, G.C.; Gendler, S.J.; Velcich, A.; Lundberg, J.O.; Roos, S.; Holm, L.; Phillipson, M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G327–G333.
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21.
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. NF-kB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158.
- Paveljšek, D.; Ivičak-Kocjan, K.; Treven, P.; Benčina, M.; Jerala, R.; Rogelj, I. Distinctive probiotic features share common TLR2-dependent signalling in intestinal epithelial cells. Cell Microbiol. 2021, 23, e13264.
- Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, Prebiotic, and Brain Development. Nutrients 2017, 9, 1247.
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035.
- Grider, J.R.; Piland, B.E. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G429–G437.
- Stefanko, D.P.; Barrett, R.M.; Ly, A.R.; Reolon, G.K.; Wood, M.A. Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci. USA 2009, 106, 9447–9452.
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G907–G915.
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605.
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 2019, 13, 1361.
- Zhang, C.X.; Wang, H.Y.; Chen, T.X. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed. Res. Int. 2019, 2019, 6764919.
- Takahashi, N.; Kitazawa, H.; Iwabuchi, N.; Xiao, J.Z.; Miyaji, K.; Iwatsuki, K.; Saito, T. Immunostimulatory oligodeoxynucleotide from Bifidobacterium longum suppresses Th2 immune responses in a murine model. Clin. Exp. Immunol. 2006, 145, 130–138.
- Kwon, H.K.; Lee, C.G.; So, J.S.; Chae, C.S.; Hwang, J.S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.C.; Im, S.H. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164.
- Liu, Y.; Rhoads, J.M. Communication between B-cells and microbiota for the maintenance of intestinal homeostasis. Antibodies 2013, 2, 535–553.
- Xu, L.Z.; Yang, L.T.; Qiu, S.Q.; Yang, G.; Luo, X.Q.; Miao, B.P.; Geng, X.R.; Liu, Z.Q.; Liu, J.; Wen, Z.; et al. Combination of specific allergen and probiotics induces specific regulatory B cells and enhances specific immunotherapy effect on allergic rhinitis. Oncotarget 2016, 7, 54360–54369.
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-kB activation in ulcerative colitis. World J. Gastroenterol. 2010, 16, 4145–4151.





