Functional gastrointestinal diseases (FGIDs) are characterized by recurring gastrointestinal symptoms, including abdominal pain, vomiting or constipation, that can ultimately cause non-optimal development, disrupt digestion or create lifelong or mortal complications. Scientific evidence suggests that both gastrointestinal diseases (GIDs) and FGIDs are related to gut microbial dysbiosis, gastrointestinal motility disturbance, visceral hypersensitivity, as well as impairment of mucosal immune function and central nervous system processing. Based on this, the use of probiotic bacteria, mainly those strains belonging to Lactobacillus, Bifidobacterium and probiotic yeast such as Saccharomyces, have emerged as potential therapeutic agents in GIDs.
1. Introduction
healthy maintenance of gut microbiota diversity can play a pivotal role in GIDs pathophysiology. Consequently, probiotics-based therapy has recently emerged as a promising strategy for the prevention and treatment of these diseases
[1][9]. Although the mechanisms of action of probiotics are complex and may differ by species, there is documented evidence on various main action pathways, including competitive adhesion and exclusion of potential pathogens, stimulation of intestinal epithelial cell (IEC) proliferation and epithelial barrier enhancement, as well as their potential interaction with the enteric nervous system and immune system (
Figure 1).
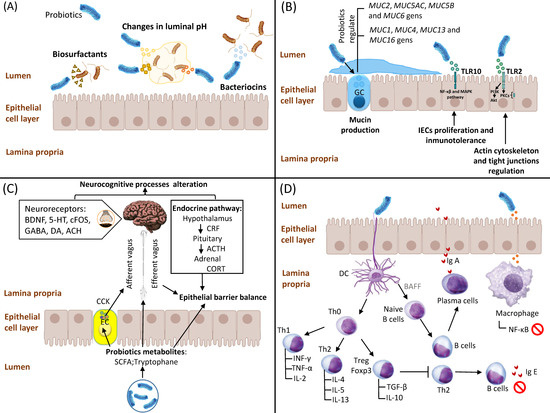
Figure 1. Mechanisms of action of probiotics. (A) Competitive adhesion and exclusion of potential pathogens. Probiotics create a hostile environment via changes in luminal pH as well as production of bacteriocins and biosurfactants. (B) Stimulation of intestinal epithelial cell (IEC) proliferation and epithelial barrier enhancement. Probiotics modulate IECs-dependent mucin production through regulation of several MUC genes. Moreover, probiotics enhance epithelial barrier and tight junctions due to their modulation of the NF-κB, MAPK PI3K/Akt and PKCs signalling pathways. (C) Interaction of probiotics and their metabolites (mainly short-chain fatty acids (SCFAs) and tryptophane) with the enteric nervous system. Probiotics modulate neurocognitive processes and epithelial barrier balance via vagus and enteric nerves; probiotics also affect the hypothalamic–pituitary–adrenal (HPA) axis via the regulation of several neurotransmitter levels, including CORT and/or ACTH, BDNF, 5-HT, c-Fos, DA, ACh and GABA. (D) Interaction of probiotics with the immune system: (i) regulation of Th1/Th2 cytokine balance and activation of CD4+ Foxp3+ Treg cells; (ii) increased levels of B cell-dependent secretory IgA (sIgA) and concomitant reduction in IgE levels; and (iii) downregulation of NF-κB signalling pathway and subsequent inhibition of inflammatory cytokines expression. Abbreviations: GC: goblet cell; PI3K: phosphatidylinositol 3-kinase; PKC: protein kinase C; EC: enteroendocrine cells; SCFA: short-chain fatty acid; CCK: cholecystokinin; CRF: corticotropin-releasing factor; ACTH: adrenocorticotropic hormone; CORT: corticosteroid; BDNF: brain-derived neurotrophic factor; 5-HT: 5 hydroxytryptamine; GABA: γ-aminobutyric acid; DA: dopamine; ACH: acetylcholine; TLR: Toll-like receptor; TNF-α: Tumor Necrosis Factor α; IFN-γ: Interferon γ; TGF-β: Transforming Growth Factor β; IL-2: Interleukin 2; IL-4: Interleukin 4; IL-5: Interleukin 5; IL-10: Interleukin 10; IL-13: Interleukin 13; Th0: T Cell Naive; Th1: Type 1 Helper T cell; Th2: Type 2 Helper T cell; IgA: immunoglobulin A; IgE: immunoglobulin E; DC: dendritic cells; BAFF: B cell-activating factor.
2. Competitive Adhesion and Exclusion of Potential Pathogens
Competitive exclusion is defined as a process by which one species of bacteria competes for the same receptor sites in the gastrointestinal tract (GIT) with other species, with identical needs for resources
[2][10]. This mechanism could enable probiotic bacteria to prevent the proliferation of potential pathogens and their adhesion to the gut epithelia. In fact, probiotics have the ability to create a hostile GIT environment to pathogenic bacteria growth through different pathways, including: (1) changes in luminal pH mediated by the secretion of short-chain fatty acids (SCFAs), branched-chain fatty acids, hydrogen sulphide and organic acids such as lactate, succinate and phenylacetate
[3][11]; (2) production of bacteriocins, mainly non-ribosomally synthesized antimicrobial peptides (e.g., gramicidin, bacitracin, polymyxin B and vancomycin)
[4][12]; and (3) production of biosurfactants (rhamnolipids and surfactin) that drastically reduce the surface tension and interfacial tension at the air–water interfaces
[5][13]. Finally, it is important to note that most bacteriocins and biosurfactants obtained from probiotic bacteria are related to a high number of lactic acid bacteria (LAB)
[6][7][14,15].
3. Stimulation of IEC Proliferation and Epithelial Barrier Enhancement
IECs are non-hematopoietic cells with pleiotropic functions in relation to luminal microbiota regulation and the host immune system, acting as a physical barrier against external antigens
[8][16]. Thus, IECs secrete a mucus coat mainly formed by glycocalyx covering the epithelial lining, which in turn creates a sheltering environment for commensal bacteria and provides an interface for immune response
[9][17]. Scientific evidence suggests that probiotics may control IEC-mediated functions through different mechanisms, including: (1) modulation of mucin production via changes in the
MUC gene expression profile
[10][18]; (2) regulation of mucosal innate immunity and subsequent production of antiapoptotic and antioxidant proteins through Toll-like receptors (TLRs)/NF-κB and MAPK signalling pathways
[11][19]; and (3) activation of TLR2s expressed in IECs, which leads to overexpression of phosphatidylinositol 3-kinase (PI3K)/Akt and protein kinase C (PKC) pathways, thus regulating the actin cytoskeleton and tight junctions in order to protect GI mucosal integrity
[12][20].
4. Interaction of Probiotics with the Enteric Nervous System
In recent years, growing evidence obtained from animal studies suggests the potential modulatory role of gut microbiota on the gut–brain axis and brain function, thus supporting the use of probiotics in the treatment and prevention of several brain and gastrointestinal diseases
[13][21]. Thus, SCFAs produced by commensal and probiotic bacteria play a pivotal role in gut microbiota–brain interactions due to their influence on the sympathetic nervous system
[14][22], mucosal serotonin release
[15][23] as well as memory and learning processes
[16][24]. Moreover, other probiotic metabolites, such as tryptophan, butyrate and oleate, can also exert their functions on the enteric nervous system through vagus and enteric nerves
[17][25]. In addition, probiotics can affect the hypothalamic–pituitary–adrenal (HPA) axis, inducing significant changes in corticosteroid (CORT) and/or adrenocorticotropic hormone (ACTH) levels. Finally, potential effects of probiotics on mind and behavior are also suggested due to the fact that their metabolism-derived compounds can modify neurotransmitters levels, including brain-derived neurotrophic factor (BDNF), 5 hydroxytryptamine (5-HT), c-Fos, dopamine (DA), acetylcholine (ACh) and γ-aminobutyric acid (GABA)
[18][26]. However, it should be noted that those neurotransmitters mentioned are also synthesized by the gut microbiota, although they are functionally different from brain-derived neurotransmitters
[19][27].
5. Interaction of Probiotics with the Immune System
Studies performed using next-generation analysis have also showed that probiotic-derived compounds regulate gene expression in immune cells and intestinal epithelium. This markedly increases immunoglobulin A (IgA) secretion by macrophages and/or dendritic cells, reduces lymphocyte polarization and cytokine profiles, as well as induces tolerance to food antigens
[20][28]. In this sense, the immunomodulatory role of probiotics is mediated by different molecular pathways, including: (i) restoration of Th1/Th2 cytokine balance through the upregulation of Th2 cytokines (IL-4, IL-5, IL-13)
[21][29], TGF-β and IL-10 production mediated by CD4+ Foxp3+ Treg cells
[22][30]; (ii) increase in B cell-dependent secretory IgA (sIgA)
[23][31] and concomitant reduction in allergen-specific IgE levels
[24][32]; and (iii) downregulation of the NF-κB signalling pathway and subsequent inhibition of inflammatory cytokines expression
[25][33]. However, these mentioned mechanisms are still not entirely clear, and further in vivo studies are needed to better understand interactions between probiotics as well as their derived compound and the immune system, which will allow us to develop novel therapeutic applications.