Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Izabella Maj | -- | 1537 | 2023-06-12 11:40:43 | | | |
| 2 | Dean Liu | -3 word(s) | 1534 | 2023-06-13 02:40:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maj, I.; Matus, K. Aluminosilicate Clay Minerals. Encyclopedia. Available online: https://encyclopedia.pub/entry/45442 (accessed on 07 February 2026).
Maj I, Matus K. Aluminosilicate Clay Minerals. Encyclopedia. Available at: https://encyclopedia.pub/entry/45442. Accessed February 07, 2026.
Maj, Izabella, Krzysztof Matus. "Aluminosilicate Clay Minerals" Encyclopedia, https://encyclopedia.pub/entry/45442 (accessed February 07, 2026).
Maj, I., & Matus, K. (2023, June 12). Aluminosilicate Clay Minerals. In Encyclopedia. https://encyclopedia.pub/entry/45442
Maj, Izabella and Krzysztof Matus. "Aluminosilicate Clay Minerals." Encyclopedia. Web. 12 June, 2023.
Copy Citation
The current focus on renewable energy sources and the circular economy favors the thermal conversion of low-quality fuels, such as biomass and waste. However, the main limitation of their usability in the power sector is the risk of slagging, fouling, ash deposition, and high-temperature corrosion. These problems may be avoided or significantly mitigated by the application of aluminosilicate clay minerals as fuel additives.
biomass
fuel
renewable energy
waste-to-energy
1. Introduction
The depletion of fossil fuels and their adverse impact on the environment, along with the unstable situation in the global energy market, has resulted in a growing interest in alternative energy sources. As presented in Table 1, the share of fossil fuels in global primary energy consumption declined from 85.9% in 2015 to 83.1% in 2020 with the simultaneous rise of renewable energy sources from 3.3% to 5.7%. Among the renewables, biomass plays a significant role as it is considered a sustainable, flexible fuel that can be used in both developed and developing countries for the transition to a low-carbon economy [1][2]. At the same time, the general amount of waste produced worldwide is growing together with economic growth and industrialization [3][4]. The total global production of waste has reached seven to nine billion tonnes per year [5], including two billion tonnes of municipal solid waste (MSW) [6]. Since not all waste can be recycled, thermal treatment is considered a sustainable method for managing mixed, contaminated, and residual MSW [7][8][9] as it provides both material utilization and energy recovery [10][11][12].
Table 1. Primary energy consumption in the world in 2015 and 2020 [13].
| Energy Source | Primary Energy Consumption in the World | |||
|---|---|---|---|---|
| 2015 | 2020 | |||
| EJ | % | EJ | % | |
| Fossil fuels | ||||
| Oil | 183.63 | 33.7 | 173.73 | 31.2 |
| Natural Gas | 125.22 | 23 | 137.62 | 24.7 |
| Coal | 158.64 | 29.1 | 151.42 | 27.2 |
| Total fossil | 467.49 | 85.9 | 462.77 | 83.1 |
| Non-Fossil fuels | ||||
| Nuclear | 23.46 | 4.3 | 23.98 | 4.3 |
| Hydro | 35.38 | 6.5 | 38.16 | 6.9 |
| Renewable | 18.1 | 3.3 | 31.71 | 5.7 |
| Total non-fossil | 76.94 | 14.1 | 93.85 | 16.9 |
| Total all sources | 544.43 | 100.0 | 556.62 | 100.0 |
Aside from unquestionable benefits, biomass and waste may be considered low-quality fuels, whose specific properties limit their usability in the power sector. The limitations are directly associated with the chemical composition of biomass and waste, most importantly the elevated contents of chlorine (Cl), sodium (Na), and potassium (K), resulting in low ash fusion temperatures (AFTs) [14][15][16][17]. Some types of farming residues may be characterized by an extreme chlorine content of above 10% [18]. Similar Cl concentrations may occur in waste since plastic and biowaste, the two main chlorine sources in MSW, comprise the main fractions of mixed waste collected from households [19]. The presence of chlorine leads to the formation of low-melting mixtures containing metal chlorides and, thus, to high-temperature corrosion through the multi-step active oxidation process [20]. Chlorine and alkalis tend to merge with silica, which occurs in the ash mainly as silica (SiO2), and reduce the fluid temperatures from approximately 1700 °C (melting point of SiO2) to approximately 750 °C. As a result of such a process, the AFTs of biomass and waste are usually lower than those of typical coals; for instance, the initial deformation temperature of biomass ash can be as low as 700 °C [21]. Such low melting tendencies lead directly to issues such as formation of deposits on heating surfaces of a boiler, slagging, fouling, high-temperature corrosion, bed agglomeration (defluidization) in circulating fluidized bed (CFB), boilers, and increased particulate matter (PM) emission [22][23][24][25][26][27][28][29], as clearly indicated by both experimental research and industrial experience [30][31][32][33]. These issues are not specific to biomass and waste but are likely to occur during the combustion of low-rank coals, as well.
Several methods for the reduction of the above problems, such as fuel mixing (blending), washing (leaching out) the unwanted elements, and the use of fuel additives have been investigated [34][35][36][37][38]. Among the additives, four main groups can be determined [39][40]:
-
Aluminosilicates-based additives;
-
Calcium-based additives;
-
Sulphur-based additives;
-
Phosphorous additives.
Among these groups, aluminosilicate clays seem particularly promising, as they are easy to handle, commonly occurring natural minerals. Their application as fuel additives may be an environmental-friendly and cost-effective remedy for operational issues of low-quality fuels. Their presence is expected to improve the ash characteristics, prevent corrosion damages, reduce particulate matter emission, and capture heavy metals in the ash. Despite the unquestionable benefits of aluminosilicates application, there is no comprehensive literature review on this field. According to the authors’ best knowledge, the available data tend to elaborate all groups of additives, and not enough attention is paid to the latest findings on aluminosilicates.
2. Characteristics of Aluminosilicate Clay Minerals
Aluminosilicate clay minerals belong to the kaolinite-serpentine subgroup and are described by the general chemical formula Al2Si2O5(OH)4. They are characterized by electroneutral layered structures with tetrahedral and octahedral sheets held together by water molecules or secondary forces such as hydrogen bonding [41]. Despite kaolin, halloysite, and bentonite, other clays such as dickite and nacrite also belong to this subgroup; however, they are less popular among industrial users. Industrial interest in kaolin, halloysite, and bentonite results from their large deposits located in Poland, as well as many other countries in the world [42][43] (Figure 1).
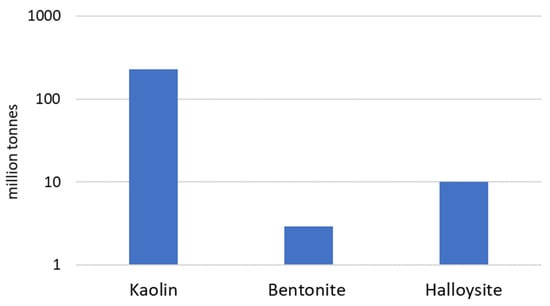
Figure 1. Aluminosilicate clay minerals deposits in Poland [43].
Aluminosilicates are suitable for diverse industrial applications, presented in Figure 2. They are commonly used as feedstock for the ceramic, chemical, and paper industries [44]. Due to their predisposition for high adsorption, they can be used as sorbents for petrochemicals, as catalysts, in cosmetics, or as dietary supplements for cattle [45][46]. Their latest application paths are composite materials [47] and biomedicine [48][49].
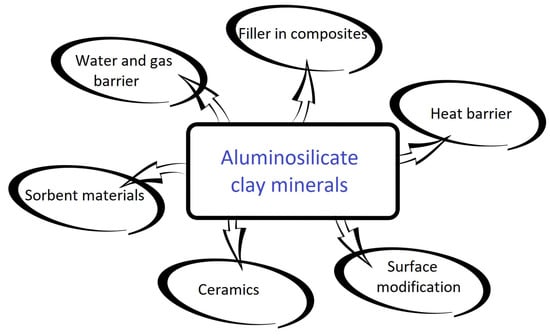
Figure 2. Common applications of aluminosilicate clay minerals.
Aluminosilicates can be considered excellent fuel additives since they meet all the specific requirements: they are characterized by high porosity and high specific surface, high reactivity, high melting points, and non-toxicity [50][51]. They are claimed to have no negative influence on the combustion process, which means they do not lower the combustion efficiency or favor the formation of any pollutants [52][53]. Their chemical stability and powdery structure make them easy to transport, store, and apply. They are characterized by low operational costs, as they occur commonly around the world, and do not require advanced pre-processing before use [42].
2.1. Halloysite
Halloysite (Al2Si2O5(OH)4·2H2O) draws the researchers’ attention due to its unique structure. Microstructural and nanostructural analysis of various halloysite samples revealed either the dominance of halloysite nanoplates (HNP), a mix of halloysite nanoplates and halloysite nanotubes (HNT), or predominantly nanotubular structure [54]. The mixed structure of halloysite consisting of both HNP and HNT is presented in Figure 3a with its chemical composition. Halloysite nanotubes are composed of two layers with mostly a hollow, slender cylindrical structure in the submicron ambit [55][56]. Among other minerals in its group, halloysite features a higher value of the specific surface area (60–80 m2/g) than the surface area of kaolin (3–12 m2/g) and the high porosity of structure. Therefore, composite materials using halloysite are becoming increasingly popular, a fascinating application of which is polyaniline-halloysite nano clay hybrid composites that can produce sensors and corrosion-resistant coatings [57]. Another potential application of halloysite is its use as a reinforcement in composites based on soy protein/basil seed gum used for the production of food packaging [58]. Finally, the potential use of halloysite also concerns materials limiting the growth of Escherichia coli bacteria [59]. Halloysite from European deposits (Figure 3b) may consist of some impurifications, mainly iron oxides, which can be considered beneficial in terms of catalytic properties [60]. The unique structural characteristics of halloysite make it the best prospective fuel additive among those considered.
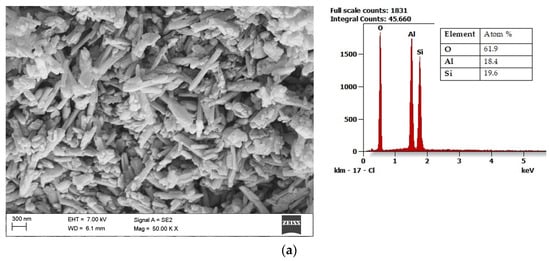
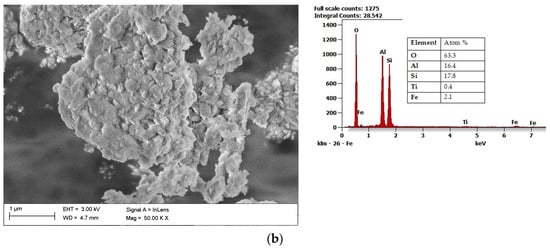
Figure 3. Scanning electron microscope (SEM) image of halloysite from USA together with results of the chemical composition analysis obtained using the Energy-dispersive (EDS) X-ray characteristic (a), SEM image of halloysite from Europe, together with results of the chemical composition analysis obtained using the EDS X-ray characteristic (b).
2.2. Kaolin
Another aluminosilicate mineral is kaolin, with a chemical composition of Al2Si2O5(OH)4. It is a layered silicate mineral, with one sheet of silica tetrahedron SiO4−4SiO44− through small atoms with one sheet of alumina octahedron Al(OH)3−6Al(OH)63−. As presented in Figure 4, it features a predominantly plate structure. Kaolin is, among all types of clay, defined as the least reactive one since it is characterized by a relatively small specific surface (3–12 m2/g) [61]. When heated, kaolin is transformed into metakaolin and further into quartz and mullite [62]. Kaolin-based slurries have a wide range of applications, such as diaphragm walls, drilling fluids, adhesives, cosmetics, refractories, and pharmaceuticals, due to their abundance in nature, low cost, and lack of swelling [63]. An increasingly popular application of kaolin is its use as an additive to composites, mainly based on polymers and biopolymers [64][65].
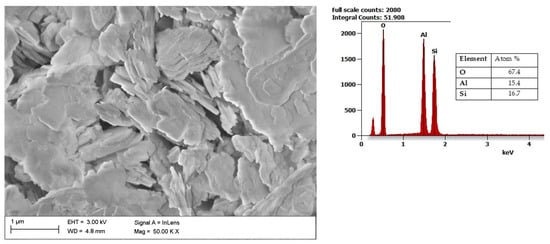
Figure 4. SEM image of Kaolin together with results of the chemical composition analysis obtained using the EDS X-ray characteristic.
2.3. Bentonite
A widely studied member of the aluminosilicates group is bentonite, which can be characterized as an absorbent swelling clay consisting mainly of montmorillonite. In addition, there is bentonite, in which there is a Na- or Ca-based montmorillonite. Similar to kaolin, bentonite features a plate structure, as presented in Figure 5. One of the main applications of bentonite is its use in the production of barrier materials [66]. The volume of the bentonite can increase several times after swelling, reducing the volume of the flow channels. Compared to other materials of this type, bentonite barriers are characterized by lower permeability and costs. Therefore, in recent years, various bentonite barriers have been developed and used [67]. The latest research focuses on the potential use of bentonite for filtration and sealing water and gas extraction processes, especially at high temperatures [68].
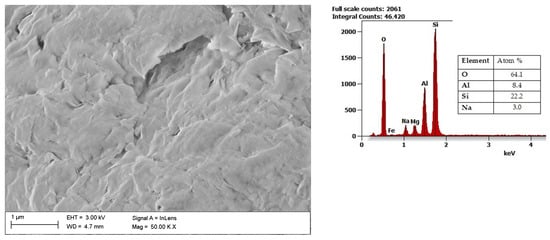
Figure 5. SEM image of bentonite with results of the chemical composition analysis obtained using the EDS X-ray characteristic.
References
- Askarova, A.; Zamorano, M.; Martín-Pascual, J.; Nugymanova, A.; Bolegenova, S. A Review of the Energy Potential of Residual Biomass for Coincineration in Kazakhstan. Energies 2022, 15, 6482.
- Li, H.; Min, X.; Dai, M.; Dong, X. The Biomass Potential and GHG (Greenhouse Gas) Emissions Mitigation of Straw-Based Biomass Power Plant: A Case Study in Anhui Province, China. Processes 2019, 7, 608.
- Chen, Y.-C. Effects of Urbanization on Municipal Solid Waste Composition. Waste Manag. 2018, 79, 828–836.
- Antczak, E. Regionally Divergent Patterns in Factors Affecting Municipal Waste Production: The Polish Perspective. Sustainability 2020, 12, 6885.
- Wilson, D.C.; Velis, C.A. Waste Management—Still a Global Challenge in the 21st Century: An Evidence-Based Call for Action. Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 1049–1051.
- The World Bank—Trends in Solid Waste Management. Available online: https://Datatopics.Worldbank.Org/What-a-Waste/Trends_in_solid_waste_management (accessed on 16 May 2023).
- Sarquah, K.; Narra, S.; Beck, G.; Bassey, U.; Antwi, E.; Hartmann, M.; Derkyi, N.S.A.; Awafo, E.A.; Nelles, M. Characterization of Municipal Solid Waste and Assessment of Its Potential for Refuse-Derived Fuel (RDF) Valorization. Energies 2022, 16, 200.
- Rajca, P.; Skibiński, A.; Biniek-Poskart, A.; Zajemska, M. Review of Selected Determinants Affecting Use of Municipal Waste for Energy Purposes. Energies 2022, 15, 9057.
- Gałko, G.; Mazur, I.; Rejdak, M.; Jagustyn, B.; Hrabak, J.; Ouadi, M.; Jahangiri, H.; Sajdak, M. Evaluation of Alternative Refuse-Derived Fuel Use as a Valuable Resource in Various Valorised Applications. Energy 2023, 263, 125920.
- Zabaniotou, A.; Vaskalis, I. Economic Assessment of Polypropylene Waste (PP) Pyrolysis in Circular Economy and Industrial Symbiosis. Energies 2023, 16, 593.
- Bourtsalas, A.C.; Shen, T.; Tian, Y. A Comprehensive Assessment of Products Management and Energy Recovery from Waste Products in the United States. Energies 2022, 15, 6581.
- Muzyka, R.; Gałko, G.; Ouadi, M.; Sajdak, M. Impact of Plastic Blends on the Gaseous Product Composition from the Co-Pyrolysis Process. Energies 2023, 16, 947.
- Holechek, J.L.; Geli, H.M.E.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792.
- Vassilev, S.v.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Fuel 2013, 105, 19–39.
- Díaz-Ramírez, M.; Frandsen, F.J.; Glarborg, P.; Sebastián, F.; Royo, J. Partitioning of K, Cl, S and P during Combustion of Poplar and Brassica Energy Crops. Fuel 2014, 134, 209–219.
- Maj, I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies 2022, 15, 8981.
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274.
- Tran, Q.T.; Maeda, M.; Oshita, K.; Takaoka, M. Phosphorus Release from Cattle Manure Ash as Soil Amendment in Laboratory-Scale Tests. Soil. Sci. Plant Nutr. 2017, 63, 369–376.
- Liikanen, M.; Sahimaa, O.; Hupponen, M.; Havukainen, J.; Sorvari, J.; Horttanainen, M. Updating and Testing of a Finnish Method for Mixed Municipal Solid Waste Composition Studies. Waste Manag. 2016, 52, 25–33.
- Kofstad, P. High Temperature Corrosion; Elsevier: London, UK, 1988.
- Vassilev, S.v.; Baxter, D.; Vassileva, C.G. An Overview of the Behaviour of Biomass during Combustion: Part II. Ash Fusion and Ash Formation Mechanisms of Biomass Types. Fuel 2014, 117, 152–183.
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189.
- Niu, Y.; Tan, H.; Hui, S. Ash-Related Issues during Biomass Combustion: Alkali-Induced Slagging, Silicate Melt-Induced Slagging (Ash Fusion), Agglomeration, Corrosion, Ash Utilization, and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61.
- Persson, K.; Broström, M.; Carlsson, J.; Nordin, A.; Backman, R. High Temperature Corrosion in a 65 MW Waste to Energy Plant. Fuel Process. Technol. 2007, 88, 1178–1182.
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382.
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211.
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The Fate of Chlorine during MSW Incineration: Vaporization, Transformation, Deposition, Corrosion and Remedies. Prog. Energy Combust. Sci. 2020, 76, 100789.
- Pronobis, M. Evaluation of the Influence of Biomass Co-Combustion on Boiler Furnace Slagging by Means of Fusibility Correlations. Biomass Bioenergy 2005, 28, 375–383.
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An Overview of Slagging and Fouling Indicators and Their Applicability to Biomass Fuels. Fuel Process. Technol. 2021, 217, 106804.
- Jiang, D.; Song, W.; Wang, X.; Zhu, Z. Physicochemical Properties of Bottom Ash Obtained from an Industrial CFB Gasifier. J. Energy Inst. 2021, 95, 375–383.
- Yao, X.; Mao, J.; Li, L.; Sun, L.; Xu, K.; Ma, X.; Hu, Y.; Zhao, Z.; Chen, S.; Xu, K. Characterization Comparison of Bottom Ash and Fly Ash during Gasification of Agricultural Residues at an Industrial-Scale Gasification Plant–Experiments and Analysis. Fuel 2021, 285, 119122.
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36.
- Zhang, H.; Yu, C.; Luo, Z.; Li, Y. Investigation of Ash Deposition Dynamic Process in an Industrial Biomass CFB Boiler Burning High-Alkali and Low-Chlorine Fuel. Energies 2020, 13, 1092.
- Singhal, A.; Konttinen, J.; Joronen, T. Effect of Different Washing Parameters on the Fuel Properties and Elemental Composition of Wheat Straw in Water-Washing Pre-Treatment. Part 2: Effect of Washing Temperature and Solid-to-Liquid Ratio. Fuel 2021, 292, 120209.
- Liu, Y.; Yan, T.; An, Y.; Zhang, W.; Dong, Y. Influence of Water Leaching on Alkali-Induced Slagging Properties of Biomass Straw. J. Fuel Chem. Technol. 2021, 49, 1839–1849.
- Suleimenova, B.; Aimbetov, B.; Zhakupov, D.; Shah, D.; Sarbassov, Y. Co-Firing of Refuse-Derived Fuel with Ekibastuz Coal in a Bubbling Fluidized Bed Reactor: Analysis of Emissions and Ash Characteristics. Energies 2022, 15, 5785.
- Wu, S.; Chen, J.; Peng, D.; Wu, Z.; Li, Q.; Huang, T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies 2019, 12, 387.
- Tsai, C.-H.; Shen, Y.-H.; Tsai, W.-T. Effect of Alkaline Pretreatment on the Fuel Properties of Torrefied Biomass from Rice Husk. Energies 2023, 16, 679.
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the Use of Additives to Mitigate Operational Problems Associated with the Combustion of Biomass with High Content in Ash-Forming Species. Renew. Sustain. Energy Rev. 2021, 141, 110502.
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29.
- Zhang, J.; Hu, L.; Pant, R.; Yu, Y.; Wei, Z.; Zhang, G. Effects of Interlayer Interactions on the Nanoindentation Behavior and Hardness of 2:1 Phyllosilicates. Appl. Clay Sci. 2013, 80, 267–280.
- Nickovic, S.; Vukovic, A.; Vujadinovic, M.; Djurdjevic, V.; Pejanovic, G. Technical Note: High-Resolution Mineralogical Database of Dust-Productive Soils for Atmospheric Dust Modeling. Atmos. Chem. Phys. 2012, 12, 845–855.
- Bilans Zasobów Złóż Kopalin W Polsce Wg Stanu Na 31 XII 2021 r; Państwowy Instytut Geologiczny—Państwowy Instytut Badawczy: Warszawa, Poland, 2022; pp. 89–90, 458–459.
- Kaolin Market Size, Share & Trends Analysis Report by Application (Paper, Ceramics, Paint & Coatings, Fiber Glass, Plastic, Rubber, Cosmetics), by Region, and Segment Forecasts, 2022–2030. Available online: https://Www.Grandviewresearch.Com/Industry-Analysis/Kaolin-Market (accessed on 15 March 2023).
- Singh, N.B. Clays and Clay Minerals in the Construction Industry. Minerals 2022, 12, 301.
- Singh, B.K.; Um, W. Application of Clay Materials for Sorption of Radionuclides from Waste Solutions. Minerals 2023, 13, 239.
- Gaaz, T.; Sulong, A.; Kadhum, A.; Al-Amiery, A.; Nassir, M.; Jaaz, A. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838.
- Nisticò, R. A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics 2022, 10, 40.
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/Halloysite Nanotubes Bionanocomposites: Structure, Mechanical Properties and Biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575.
- Murray, H.H. Applied Clay Mineralogy Today and Tomorrow. Clay Miner. 1999, 34, 39–49.
- Bergaya, F.; Lagaly, G. Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science. Dev. Clay Sci. 2006, 1, 1–18.
- Wejkowski, R.; Kalisz, S.; Tymoszuk, M.; Ciukaj, S.; Maj, I. Full-Scale Investigation of Dry Sorbent Injection for NOx Emission Control and Mercury Retention. Energies 2021, 14, 7787.
- Kalisz, S.; Ciukaj, S.; Mroczek, K.; Tymoszuk, M.; Wejkowski, R.; Pronobis, M.; Kubiczek, H. Full-Scale Study on Halloysite Fireside Additive in 230 t/h Pulverized Coal Utility Boiler. Energy 2015, 92, 33–39.
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670.
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and Properties of Natural Nanocomposites Based on Natural Rubber and Naturally Occurring Halloysite Nanotubes. Mater. Des. 2010, 31, 2151–2156.
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of Halloysite, Imogolite, and Boron Nitride Nanotubes in Solvent Media. Appl. Sci. 2018, 8, 1068.
- Prabhu, R.; Shetty, K.; Jeevananda, T.; Ananda Murthy, H.C.; Sillanpaa, M.; Nhat, T. Novel Polyaniline–Halloysite Nanoclay Hybrid Composites: Synthesis, Physico-Chemical, Thermal and Electrical Properties. Inorg. Chem. Commun. 2023, 148, 110328.
- Shahabi, N.; Soleimani, S.; Ghorbani, M. Investigating Functional Properties of Halloysite Nanotubes and Propolis Used in Reinforced Composite Film Based on Soy Protein/Basil Seed Gum for Food Packaging Application. Int. J. Biol. Macromol. 2023, 231, 123350.
- Alekseeva, O.V.; Smirnova, D.N.; Noskov, A.V.; Kuznetsov, O.Y.; Kirilenko, M.A.; Agafonov, A.V. Mesoporous Halloysite/Magnetite Composite: Synthesis, Characterization and in Vitro Evaluation of the Effect on the Bacteria Viability. Mater. Today Commun. 2022, 32, 103877.
- Sakiewicz, P.; Lutyński, M.; Sołtys, J.; Pytliński, A. Purification of Halloysite by Magnetic Separation. Physicochem. Probl. Miner. Process. 2016, 52, 991–1001.
- Ciftbudak, S.; Kalkan, B.; Bozbay, R.; Er, M.; Orakdogen, N. Structure-Property Relationships of Kaolin-Nanocomposite Beads Decorated with Tertiary Amines: Influence of Shape on Network Elasticity and Multi-Responsivity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130471.
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal Dehydroxylation of Kaolinite under Isothermal Conditions. Appl. Clay Sci. 2013, 80, 417–425.
- Murray, H.H. Chapter 5 Kaolin Applications. Dev. Clay Sci. 2006, 2, 85–109.
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73.
- Shakeel, A.; Ali, W.; Chassagne, C.; Kirichek, A. Tuning the Rheological Properties of Kaolin Suspensions Using Biopolymers. Colloids. Surf. A Physicochem. Eng. Asp. 2022, 654, 130120.
- Cui, Q.; Chen, B. Review of Polymer-Amended Bentonite: Categories, Mechanism, Modification Processes and Application in Barriers for Isolating Contaminants. Appl. Clay Sci. 2023, 235, 106869.
- Alzamel, M.; Fall, M.; Haruna, S. Swelling Ability and Behaviour of Bentonite-Based Materials for Deep Repository Engineered Barrier Systems: Influence of Physical, Chemical and Thermal Factors. J. Rock Mech. Geotech. Eng. 2022, 14, 689–702.
- Zhong, H.; Guan, Y.; Qiu, Z.; Grady, B.P.; Su, J.; Huang, W. Application of Carbon Coated Bentonite Composite as an Ultra-High Temperature Filtration Reducer in Water-Based Drilling Fluid. J. Mol. Liq. 2023, 375, 121360.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
13 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




