Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Izabella Maj and Version 2 by Dean Liu.
The current focus on renewable energy sources and the circular economy favors the thermal conversion of low-quality fuels, such as biomass and waste. However, the main limitation of their usability in the power sector is the risk of slagging, fouling, ash deposition, and high-temperature corrosion. These problems may be avoided or significantly mitigated by the application of aluminosilicate clay minerals as fuel additives.
- biomass
- fuel
- renewable energy
- waste-to-energy
1. Introduction
The depletion of fossil fuels and their adverse impact on the environment, along with the unstable situation in the global energy market, has resulted in a growing interest in alternative energy sources. As presented in Table 1, the share of fossil fuels in global primary energy consumption declined from 85.9% in 2015 to 83.1% in 2020 with the simultaneous rise of renewable energy sources from 3.3% to 5.7%. Among the renewables, biomass plays a significant role as it is considered a sustainable, flexible fuel that can be used in both developed and developing countries for the transition to a low-carbon economy [1][2][1,2]. At the same time, the general amount of waste produced worldwide is growing together with economic growth and industrialization [3][4][3,4]. The total global production of waste has reached seven to nine billion tonnes per year [5], including two billion tonnes of municipal solid waste (MSW) [6]. Since not all waste can be recycled, thermal treatment is considered a sustainable method for managing mixed, contaminated, and residual MSW [7][8][9][7,8,9] as it provides both material utilization and energy recovery [10][11][12][10,11,12].
| Energy Source | Primary Energy Consumption in the World | |||
|---|---|---|---|---|
| 2015 | 2020 | |||
| EJ | % | EJ | % | |
| Fossil fuels | ||||
| Oil | 183.63 | 33.7 | 173.73 | 31.2 |
| Natural Gas | 125.22 | 23 | 137.62 | 24.7 |
| Coal | 158.64 | 29.1 | 151.42 | 27.2 |
| Total fossil | 467.49 | 85.9 | 462.77 | 83.1 |
| Non-Fossil fuels | ||||
| Nuclear | 23.46 | 4.3 | 23.98 | 4.3 |
| Hydro | 35.38 | 6.5 | 38.16 | 6.9 |
| Renewable | 18.1 | 3.3 | 31.71 | 5.7 |
| Total non-fossil | 76.94 | 14.1 | 93.85 | 16.9 |
| Total all sources | 544.43 | 100.0 | 556.62 | 100.0 |
Aside from unquestionable benefits, biomass and waste may be considered low-quality fuels, whose specific properties limit their usability in the power sector. The limitations are directly associated with the chemical composition of biomass and waste, most importantly the elevated contents of chlorine (Cl), sodium (Na), and potassium (K), resulting in low ash fusion temperatures (AFTs) [14][15][16][17][14,15,16,17]. Some types of farming residues may be characterized by an extreme chlorine content of above 10% [18]. Similar Cl concentrations may occur in waste since plastic and biowaste, the two main chlorine sources in MSW, comprise the main fractions of mixed waste collected from households [19]. The presence of chlorine leads to the formation of low-melting mixtures containing metal chlorides and, thus, to high-temperature corrosion through the multi-step active oxidation process [20]. Chlorine and alkalis tend to merge with silica, which occurs in the ash mainly as silica (SiO2), and reduce the fluid temperatures from approximately 1700 °C (melting point of SiO2) to approximately 750 °C. As a result of such a process, the AFTs of biomass and waste are usually lower than those of typical coals; for instance, the initial deformation temperature of biomass ash can be as low as 700 °C [21]. Such low melting tendencies lead directly to issues such as formation of deposits on heating surfaces of a boiler, slagging, fouling, high-temperature corrosion, bed agglomeration (defluidization) in circulating fluidized bed (CFB), boilers, and increased particulate matter (PM) emission [22][23][24][25][26][27][28][29][22,23,24,25,26,27,28,29], as clearly indicated by both experimental research and industrial experience [30][31][32][33][30,31,32,33]. These issues are not specific to biomass and waste but are likely to occur during the combustion of low-rank coals, as well.
Several methods for the reduction of the above problems, such as fuel mixing (blending), washing (leaching out) the unwanted elements, and the use of fuel additives have been investigated [34][35][36][37][38][34,35,36,37,38]. Among the additives, four main groups can be determined [39][40][39,40]:
-
Aluminosilicates-based additives;
-
Calcium-based additives;
-
Sulphur-based additives;
-
Phosphorous additives.
Among these groups, aluminosilicate clays seem particularly promising, as they are easy to handle, commonly occurring natural minerals. Their application as fuel additives may be an environmental-friendly and cost-effective remedy for operational issues of low-quality fuels. Their presence is expected to improve the ash characteristics, prevent corrosion damages, reduce particulate matter emission, and capture heavy metals in the ash. Despite the unquestionable benefits of aluminosilicates application, there is no comprehensive literature review on this field. According to the authors’ best knowledge, the available data tend to elaborate all groups of additives, and not enough attention is paid to the latest findings on aluminosilicates.
2. Characteristics of Aluminosilicate Clay Minerals
Aluminosilicate clay minerals belong to the kaolinite-serpentine subgroup and are described by the general chemical formula Al2Si2O5(OH)4. They are characterized by electroneutral layered structures with tetrahedral and octahedral sheets held together by water molecules or secondary forces such as hydrogen bonding [41]. Despite kaolin, halloysite, and bentonite, other clays such as dickite and nacrite also belong to this subgroup; however, they are less popular among industrial users. Industrial interest in kaolin, halloysite, and bentonite results from their large deposits located in Poland, as well as many other countries in the world [42][43][42,43] (Figure 1).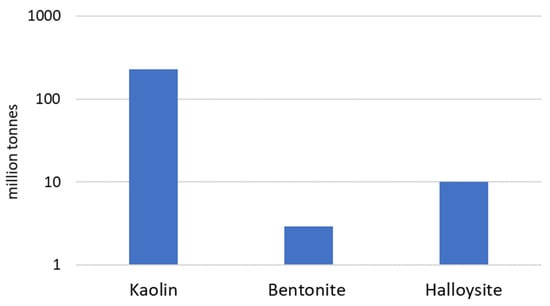
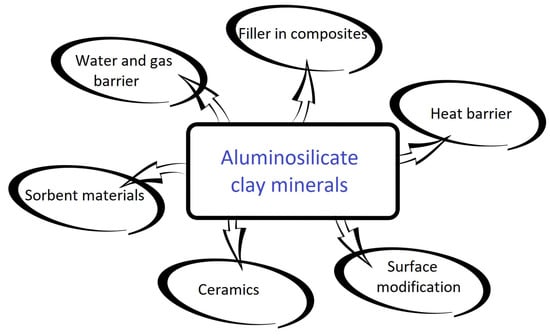
Figure 2. Common applications of aluminosilicate clay minerals.
2.1. Halloysite
Halloysite (Al2Si2O5(OH)4·2H2O) draws the researchers’ attention due to its unique structure. Microstructural and nanostructural analysis of various halloysite samples revealed either the dominance of halloysite nanoplates (HNP), a mix of halloysite nanoplates and halloysite nanotubes (HNT), or predominantly nanotubular structure [54]. The mixed structure of halloysite consisting of both HNP and HNT is presented in Figure 3a with its chemical composition. Halloysite nanotubes are composed of two layers with mostly a hollow, slender cylindrical structure in the submicron ambit [55][56][55,56]. Among other minerals in its group, halloysite features a higher value of the specific surface area (60–80 m2/g) than the surface area of kaolin (3–12 m2/g) and the high porosity of structure. Therefore, composite materials using halloysite are becoming increasingly popular, a fascinating application of which is polyaniline-halloysite nano clay hybrid composites that can produce sensors and corrosion-resistant coatings [57]. Another potential application of halloysite is its use as a reinforcement in composites based on soy protein/basil seed gum used for the production of food packaging [58]. Finally, the potential use of halloysite also concerns materials limiting the growth of Escherichia coli bacteria [59]. Halloysite from European deposits (Figure 3b) may consist of some impurifications, mainly iron oxides, which can be considered beneficial in terms of catalytic properties [60]. The unique structural characteristics of halloysite make it the best prospective fuel additive among those considered in this study.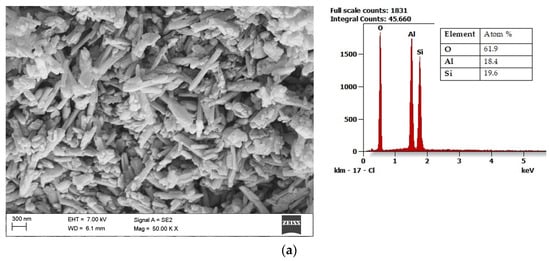
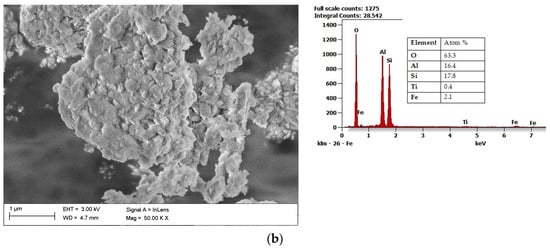
Figure 3. Scanning electron microscope (SEM) image of halloysite from USA together with results of the chemical composition analysis obtained using the Energy-dispersive (EDS) X-ray characteristic (a), SEM image of halloysite from Europe, together with results of the chemical composition analysis obtained using the EDS X-ray characteristic (b).
2.2. Kaolin
Another aluminosilicate mineral is kaolin, with a chemical composition of Al2Si2O5(OH)4. It is a layered silicate mineral, with one sheet of silica tetrahedron SiO4−4SiO44− through small atoms with one sheet of alumina octahedron Al(OH)3−6Al(OH)63−. As presented in Figure 4, it features a predominantly plate structure. Kaolin is, among all types of clay, defined as the least reactive one since it is characterized by a relatively small specific surface (3–12 m2/g) [61]. When heated, kaolin is transformed into metakaolin and further into quartz and mullite [62]. Kaolin-based slurries have a wide range of applications, such as diaphragm walls, drilling fluids, adhesives, cosmetics, refractories, and pharmaceuticals, due to their abundance in nature, low cost, and lack of swelling [63]. An increasingly popular application of kaolin is its use as an additive to composites, mainly based on polymers and biopolymers [64][65][64,65].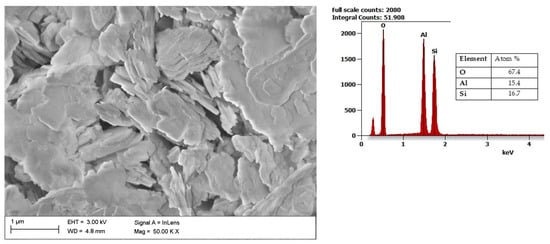
Figure 4. SEM image of Kaolin together with results of the chemical composition analysis obtained using the EDS X-ray characteristic.
2.3. Bentonite
A widely studied member of the aluminosilicates group is bentonite, which can be characterized as an absorbent swelling clay consisting mainly of montmorillonite. In addition, there is bentonite, in which there is a Na- or Ca-based montmorillonite. Similar to kaolin, bentonite features a plate structure, as presented in Figure 5. One of the main applications of bentonite is its use in the production of barrier materials [66]. The volume of the bentonite can increase several times after swelling, reducing the volume of the flow channels. Compared to other materials of this type, bentonite barriers are characterized by lower permeability and costs. Therefore, in recent years, various bentonite barriers have been developed and used [67]. The latest research focuses on the potential use of bentonite for filtration and sealing water and gas extraction processes, especially at high temperatures [68].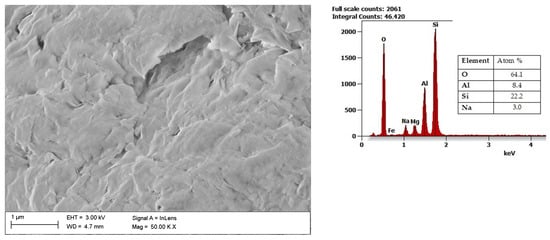
Figure 5. SEM image of bentonite with results of the chemical composition analysis obtained using the EDS X-ray characteristic.
