
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasile Valeriu Lupu | -- | 3507 | 2023-05-24 09:47:25 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 3508 | 2023-05-24 11:23:56 | | |
Video Upload Options
Heart failure (HF) is a worldwide health problem with important consequences for the overall wellbeing of affected individuals as well as for the healthcare system. Numerous pieces of evidence have demonstrated that the associated gut microbiota represent an important component of human physiology and metabolic homeostasis, and can affect one’s state of health or disease directly, or through their derived metabolites. The advances in human microbiome studies shed light on the relationship between the gut microbiota and the cardiovascular system, revealing its contribution to the development of heart failure-associated dysbiosis. HF has been linked to gut dysbiosis, low bacterial diversity, intestinal overgrowth of potentially pathogenic bacteria and a decrease in short chain fatty acids-producing bacteria. An increased intestinal permeability allowing microbial translocation and the passage of bacterial-derived metabolites into the bloodstream is associated with HF progression. A more insightful understanding of the interactions between the human gut microbiome, HF and the associated risk factors is mandatory for optimizing therapeutic strategies based on microbiota modulation and offering individualized treatment.
1. Introduction
2. Gut-Associated Microbiome Composition and Function in Healthy Individuals
3. Impaired Gut Barrier Function and Inflammation in Heart Failure
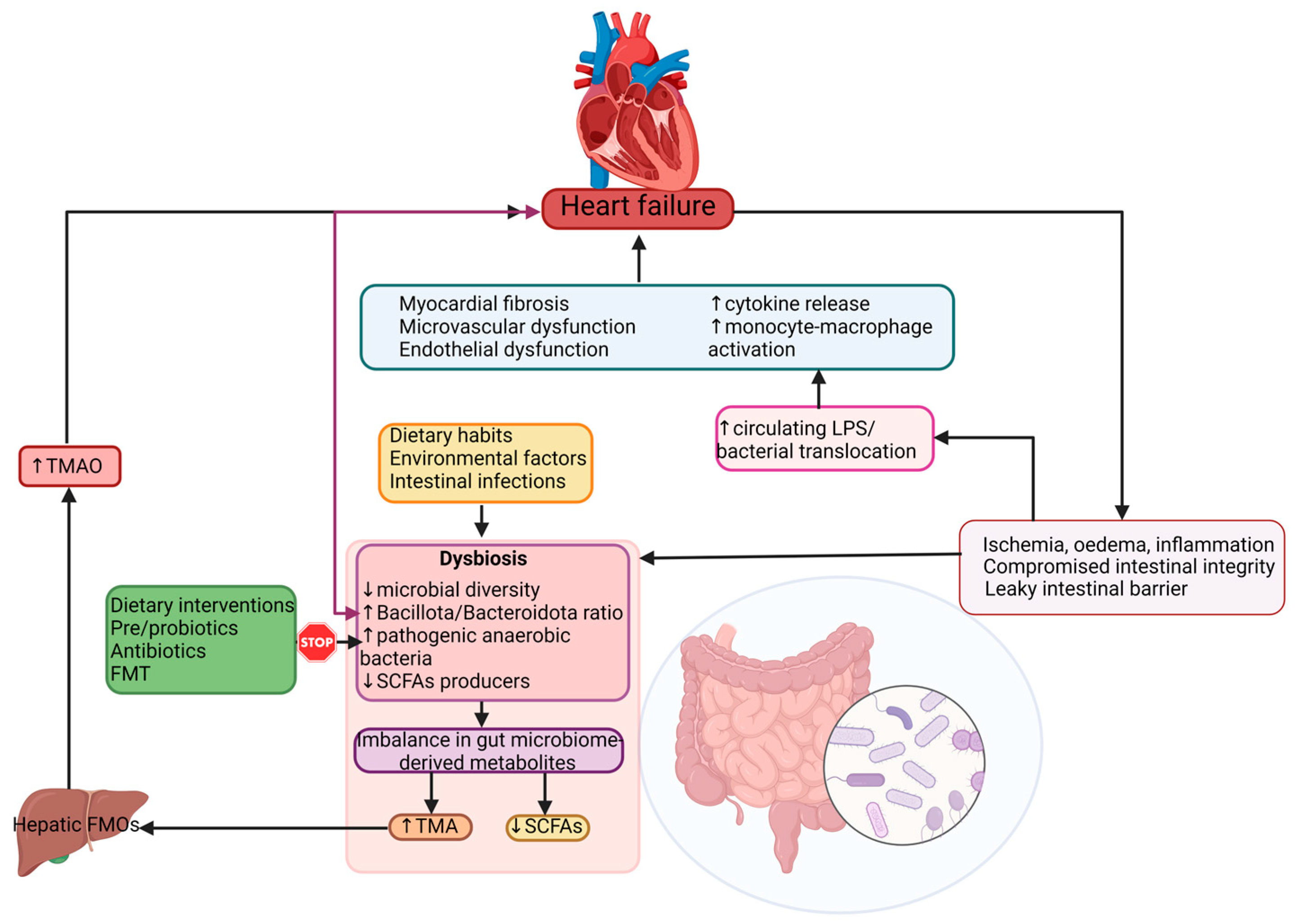
4. Dysbiosis in Heart Failure
5. Risk Factors for HF and Gut Microbiota
5.1. Dietary Choices
5.2. Obesity
5.3. Type II Diabetes Mellitus
5.4. Hypertension
6. Gut-Derived Metabolites as Possible Biomarkers Related to Intestinal Dysbiosis in HF
7. Interactions between the Gut Microbiome and Cardiovascular Drugs
7.1. Cardiac Glycosides
7.2. Blood Thinners and Gut Microbiota
7.3. The Effects of Beta-Blockers, ACEi, and ARBs on Gut Microbiota
7.4. Statins and Gut Microbiota
8. Modulation of Dysbiosis as a Potential Target in Heart Failure
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356.
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154.
- Shirazi, L.F.; Bissett, J.; Romeo, F.; Mehta, J.L. Role of Inflammation in Heart Failure. Curr. Atheroscler. Rep. 2017, 19, 27.
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22.
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186.
- Hietbrink, F.; Besselink, M.G.; Renooij, W.; de Smet, M.B.; Draisma, A.; van der Hoeven, H.; Pickkers, P. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock 2009, 32, 374–378.
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117.
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836.
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180, Erratum in Nature 2011, 474, 666; Erratum in Nature 2014, 506, 516.
- Sharon, I.; Quijada, N.M.; Pasolli, E.; Fabbrini, M.; Vitali, F.; Agamennone, V.; Dötsch, A.; Selberherr, E.; Grau, J.H.; Meixner, M.; et al. The Core Human Microbiome: Does It Exist and How Can We Find It? A Critical Review of the Concept. Nutrients 2022, 14, 2872.
- Sandek, A.; Bjarnason, I.; Volk, H.D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012, 157, 80–85.
- Piggott, D.A.; Tuddenham, S. The gut microbiome and frailty. Transl. Res. 2020, 221, 23–43.
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The Not-so-Sterile Womb: Evidence That the Human Fetus Is Exposed to Bacteria Prior to Birth. Front. Microbiol. 2019, 10, 1124.
- Li, S.; Kararigas, G. Role of Biological Sex in the Cardiovascular-Gut Microbiome Axis. Front. Cardiovasc. Med. 2022, 8, 759735.
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Wang, W.; Zhu, L.J.; Leng, Y.Q.; Wang, Y.W.; Shi, T.; Wang, W.Z.; Sun, J.C. Inflammatory Response: A Crucial Way for Gut Microbes to Regulate Cardiovascular Diseases. Nutrients 2023, 15, 607.
- Steimle, A.; Frick, J.S. Molecular Mechanisms of Induction of Tolerant and Tolerogenic Intestinal Dendritic Cells in Mice. J. Immunol. Res. 2016, 2016, 1958650.
- Ilyas, A.; Wijayasinghe, Y.S.; Khan, I. Implications of trimethylamine N-oxide (TMAO) and Betaine in Human Health: Beyond Being Osmoprotective Compounds. Front. Mol. Biosci. 2022, 9, 964624.
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296.
- Nagatomo, Y.; Tang, W.H.W. Intersections between microbiome and heart failure: Revisiting the gut hypothesis. J. Card Fail. 2015, 21, 973–980.
- Mu, F.; Tang, M.; Guan, Y.; Lin, R.; Zhao, M.; Zhao, J.; Huang, S.; Zhang, H.; Wang, J.; Tang, H. Knowledge Mapping of the Links Between the Gut Microbiota and Heart Failure: A Scientometric Investigation (2006–2021). Front. Cardiovasc. Med. 2022, 9, 882660.
- Kamo, T.; Akazawa, H.; Suda, W.; Saga-Kamo, A.; Shimizu, Y.; Yagi, H.; Liu, Q.; Nomura, S.; Naito, A.T.; Takeda, N.; et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE 2017, 12, e0174099.
- Gallo, A.; Macerola, N.; Favuzzi, A.M.; Nicolazzi, M.A.; Gasbarrini, A.; Montalto, M. The Gut in Heart Failure: Current Knowledge and Novel Frontiers. Med. Princ. Pract. 2022, 31, 203–214.
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1110.
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569.
- Anker, S.D.; Egerer, K.R.; Volk, H.D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am. J. Cardiol. 1997, 79, 1426–1430.
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151.
- Peschel, T.; Schonauer, M.; Thiele, H.; Anker, S.D.; Schuler, G.; Niebauer, J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur. J. Heart Fail 2003, 5, 609–614.
- Thierer, J.; Acosta, A.; Vainstein, N.; Sultan, M.; Francesia, A.; Marino, J.; Prado, A.H.; Guglielmone, R.; Trivi, M.; Boero, L.; et al. Relation of left ventricular ejection fraction and functional capacity with metabolism and inflammation in chronic heart failure with reduced ejection fraction (from the MIMICA Study). Am. J. Cardiol. 2010, 105, 977–983.
- Deswal, A.; Petersen, N.J.; Feldman, A.M.; Young, J.B.; White, B.G.; Mann, D.L. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001, 103, 2055–2059.
- Rauchhaus, M.; Doehner, W.; Francis, D.P.; Davos, C.; Kemp, M.; Liebenthal, C.; Niebauer, J.; Hooper, J.; Volk, H.D.; Coats, A.J.; et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 2000, 102, 3060–3067.
- Kitai, T.; Kirsop, J.; Tang, W.H. Exploring the Microbiome in Heart Failure. Curr. Heart Fail Rep. 2016, 13, 103–109.
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227.
- Hayashi, T.; Yamashita, T.; Watanabe, H.; Kami, K.; Yoshida, N.; Tabata, T.; Emoto, T.; Sasaki, N.; Mizoguchi, T.; Irino, Y.; et al. Gut Microbiome and Plasma Microbiome-Related Metabolites in Patients With Decompensated and Compensated Heart Failure. Circ. J. 2018, 83, 182–192.
- Sun, W.; Du, D.; Fu, T.; Han, Y.; Li, P.; Ju, H. Alterations of the Gut Microbiota in Patients With Severe Chronic Heart Failure. Front. Microbiol. 2022, 12, 813289.
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923.
- Cui, X.; Ye, L.; Li, J.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; Zhou, X.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635.
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736.
- Martin, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67.
- Leonel, A.J.; Alvarez-Leite, J.I. Butyrate: Implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 474–479.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Li, L.; Zhong, S.J.; Hu, S.Y.; Cheng, B.; Qiu, H.; Hu, Z.X. Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol. 2021, 21, 141.
- Yu, W.; Jiang, Y.; Xu, H.; Zhou, Y. The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies. Biomedicines 2023, 11, 442.
- Hairrman, R.S.; Gouveia, C.G.; Sichinel, Â.H.; Silva, L.S.A.; Oliveira, T.S.S.; Farias, M.N. Tmao and the relationship with cardiovascular disease: The elderly and their physiological aspects. Braz. J. Dev. 2021, 7, 6971–6982.
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649.
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914.
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709.
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271.
- Wang, Y.C.; Liang, C.S.; Gopal, D.M.; Ayalon, N.; Donohue, C.; Santhanakrishnan, R.; Sandhu, H.; Perez, A.J.; Downing, J.; Gokce, N.; et al. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ. Heart Fail. 2015, 8, 897–904.
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590.
- Okamura, M.; Ueno, T.; Tanaka, S.; Murata, Y.; Kobayashi, H.; Miyamoto, A.; Abe, M.; Fukuda, N. Increased expression of acyl-CoA oxidase 2 in the kidney with plasma phytanic acid and altered gut microbiota in spontaneously hypertensive rats. Hypertens Res. 2021, 44, 651–661.
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340.
- Adnan, S.; Nelson, J.W.; Ajami, N.J.; Venna, V.R.; Petrosino, J.F.; Bryan, R.M., Jr.; Durgan, D.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 2017, 49, 96–104.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 3599–3726.
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in Circulation 2022, 145, e1033; Erratum in Circulation 2022, 146, e185.
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982.
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850.
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Renal. Physiol. 2019, 316, F1211–F1217.
- Francisqueti-Ferron, F.V.; Nakandakare-Maia, E.T.; Siqueira, J.S.; Ferron, A.J.T.; Vieira, T.A.; Bazan, S.G.Z.; Corrêa, C.R. The role of gut dysbiosis-associated inflammation in heart failure. Rev. Assoc. Med. Bras 2022, 68, 1120–1124.
- Tuerhongjiang, G.; Guo, M.; Qiao, X.; Lou, B.; Wang, C.; Wu, H.; Wu, Y.; Yuan, Z.; She, J. Interplay Between Gut Microbiota and Amino Acid Metabolism in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 752241.
- Chen, W.S.; Wang, C.H.; Cheng, C.W.; Liu, M.H.; Chu, C.M.; Wu, H.P.; Huang, P.C.; Lin, Y.T.; Ko, T.; Chen, W.H.; et al. Elevated plasma phenylalanine predicts mortality in critical patients with heart failure. ESC Heart Fail. 2020, 7, 2884–2893.
- Chen, X.; Li, H.Y.; Hu, X.M.; Zhang, Y.; Zhang, S.Y. Current understanding of gut microbiota alterations and related therapeutic intervention strategies in heart failure. Chin. Med. J. 2019, 132, 1843–1855.
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.; Org, E.; Sheehy, B.; Britt, E.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585.
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595.
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell. Cardiol. 2019, 134, 119–130.
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82.
- Salzano, A.; Cassambai, S.; Yazaki, Y.; Israr, M.Z.; Bernieh, D.; Wong, M.; Suzuki, T. The Gut Axis Involvement in Heart Failure: Focus on Trimethylamine N-oxide. Cardiol. Clin. 2022, 40, 161–169.
- Xu, J.; Yang, Y. Gut microbiome and its meta-omics perspectives: Profound implications for cardiovascular diseases. Gut Microbes. 2021, 13, 1936379.
- Guan, X.; Sun, Z. The Role of Intestinal Flora and Its Metabolites in Heart Failure. Infect. Drug Resist. 2023, 16, 51–64.
- Mayerhofer, C.C.K.; Kummen, M.; Holm, K.; Broch, K.; Awoyemi, A.; Vestad, B.; Storm-Larsen, C.; Seljeflot, I.; Ueland, T.; Bohov, P.; et al. Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Fail. 2020, 7, 456–466.
- Tuteja, S.; Ferguson, J.F. Gut Microbiome and Response to Cardiovascular Drugs. Circ. Genom. Precis Med. 2019, 12, 421–429.
- Kim, D.-H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab. Dispos. 2015, 43, 1581–1589.
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25.
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569.
- Jackson, M.A.; Verdi, S.; Maxan, M.E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655.
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium eggerthella lenta. Science 2013, 341, 295–298.
- Saha, J.R.; Butler, V.P., Jr.; Neu, H.C.; Lindenbaum, J. Digoxin-inactivating bacteria: Identification in human gut flora. Science 1983, 220, 325–327.
- Kim, I.S.; Yoo, D.H.; Jung, I.H.; Lim, S.; Jeong, J.J.; Kim, K.A.; Bae, O.N.; Yoo, H.H.; Kim, D.H. Reduced metabolic activity of gut microbiota by antibiotics can potentiate the antithrombotic effect of aspirin. Biochem. Pharmacol. 2016, 122, 72–79.
- Mortensen, B.; Murphy, C.; O’Grady, J.; Lucey, M.; Elsafi, G.; Barry, L.; Westphal, V.; Wellejus, A.; Lukjancenko, O.; Eklund, A.; et al. Bifidobacteriumbreve Bif195 Protects Against Small-Intestinal Damage Caused by Acetylsalicylic Acid in Healthy Volunteers. Gastroenterology 2019, 157, 637–646.e4.
- Liu, Y.; Song, X.; Zhou, H.; Zhou, X.; Xia, Y.; Dong, X.; Zhong, W.; Tang, S.; Wang, L.; Wen, S.; et al. Gut microbiome associates with lipid-lowering effect of rosuvastatin in vivo. Front. Microbiol. 2018, 9, 530.
- Khan, T.J.; Ahmed, Y.M.; Zamzami, M.A.; Siddiqui, A.M.; Khan, I.; Baothman, O.A.S.; Mehanna, M.G.; Kuerban, A.; Kaleemuddin, M.; Yasir, M. Atorvastatin treatment modulates the gut microbiota of the hypercholesterolemic patients. Omics J. Integr. Biol. 2018, 22, 154–163.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7.
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821.
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014, 99, 172–180.
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977.
- Mamic, P.; Chaikijurajai, T.; Tang, W.H.W. Gut microbiome—A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J. Mol. Cell Cardiol. 2021, 152, 105–117.
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012, 26, 1727–1735.
- Ponziani, F.R.; Zocco, M.A.; D’Aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J. Gastroenterol. 2017, 23, 4491–4499.
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215.
- Conraads, V.M.; Jorens, P.G.; De Clerck, L.S.; Van Saene, H.K.; Ieven, M.M.; Bosmans, J.M.; Schuerwegh, A.; Bridts, C.H.; Wuyts, F.; Stevens, W.J.; et al. Selective intestinal decontamination in advanced chronic heart failure: A pilot trial. Eur. J. Heart Fail. 2004, 6, 483–491.
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92.
- Miao, T.; Yu, Y.; Sun, J.; Ma, A.; Yu, J.; Cui, M.; Yang, L.; Wang, H. Decrease in abundance of bacteria of the genus Bifidobacterium in gut microbiota may be related to pre-eclampsia progression in women from East China. Food Nutr. Res. 2021, 65.





