
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jennie Han | -- | 2465 | 2023-05-17 00:58:57 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2465 | 2023-05-17 02:55:12 | | |
Video Upload Options
The benefits of exercise for cardiovascular and general health are many. However, sudden cardiac death (SCD) may occur in apparently healthy athletes who perform at the highest levels. A diverse spectrum of diseases is implicated in SCD in athletes, and while atherosclerotic coronary artery disease predominates in individuals of >35 years of age, primary cardiomyopathies and ion channelopathies are prevalent in young individuals. Prevention of SCD in athletes relies on the implementation of health policies aimed at the early identification of arrhythmogenic diseases (such as cardiac screening) and successful resuscitation (such as widespread utilization of automatic external defibrillators and training members of the public on cardiopulmonary resuscitation).
1. Causes of Sudden Cardiac Death
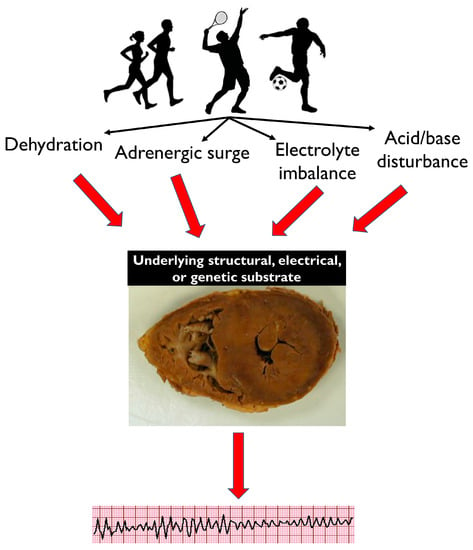
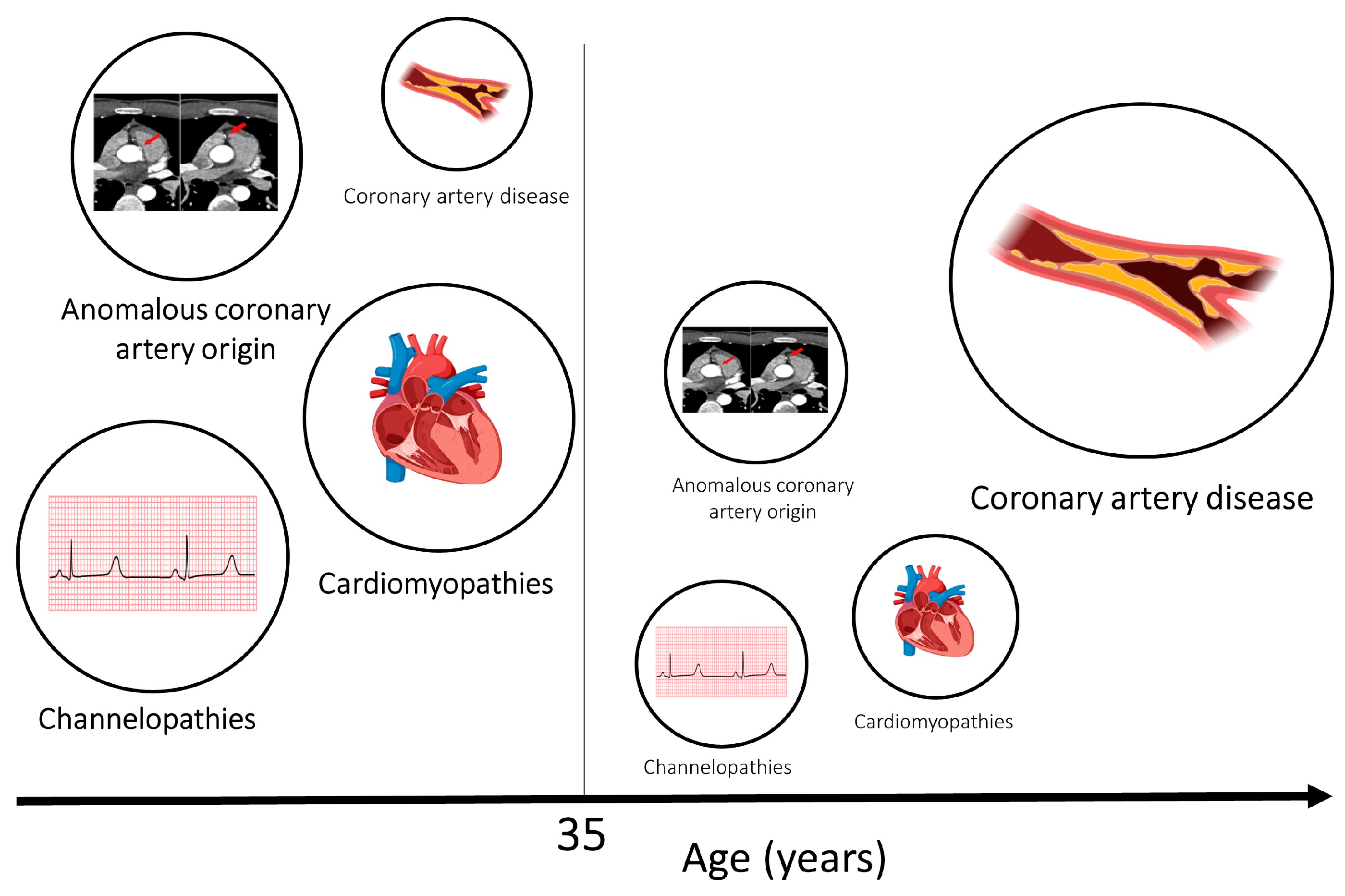
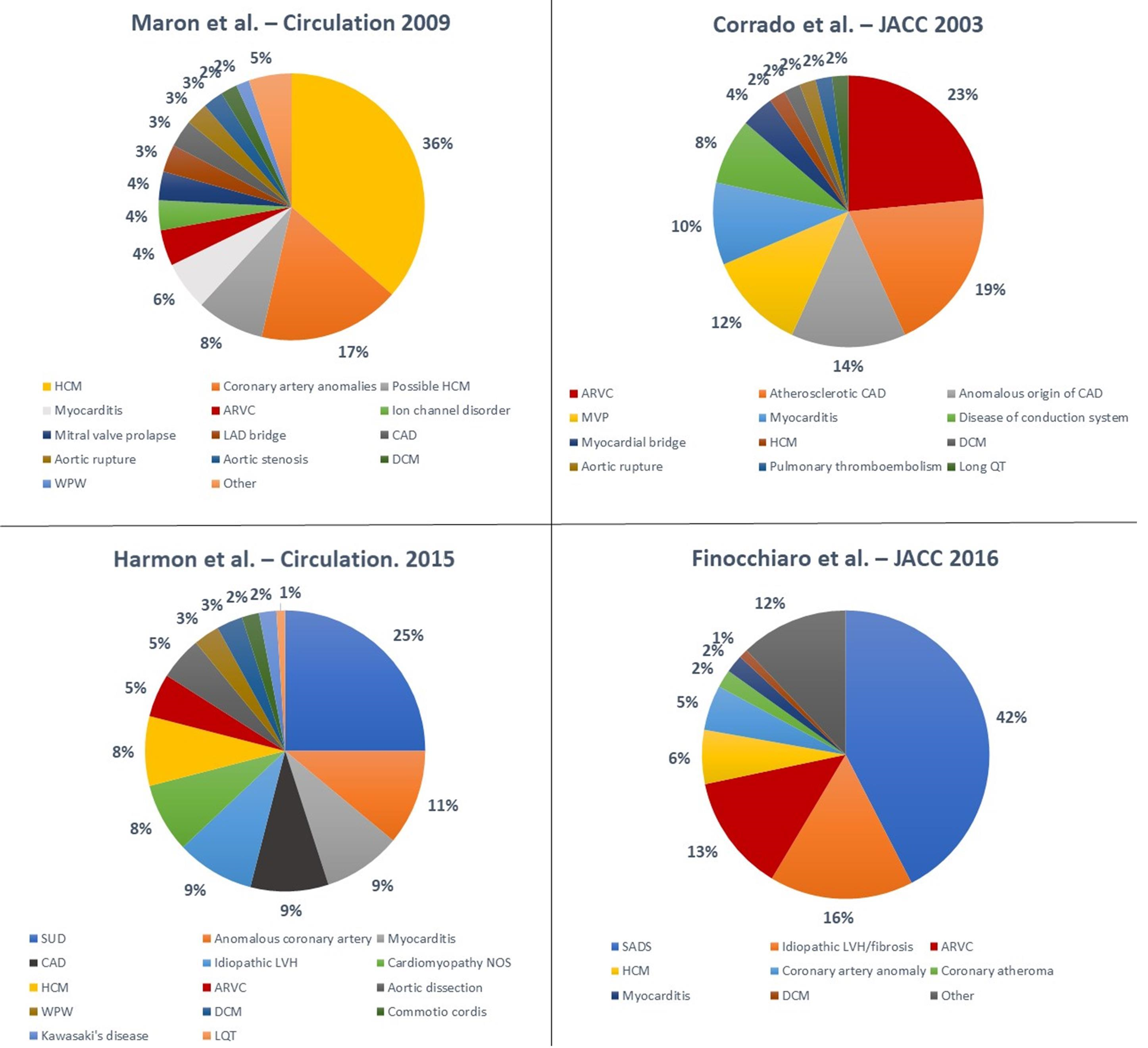
Circumstances of Death
2. Prevention of SCD in Athletes
2.1. Pre-Participation Cardiac Screening
2.2. Differential Diagnosis between “Athlete’s Heart” and Pathological Cardiac Conditions
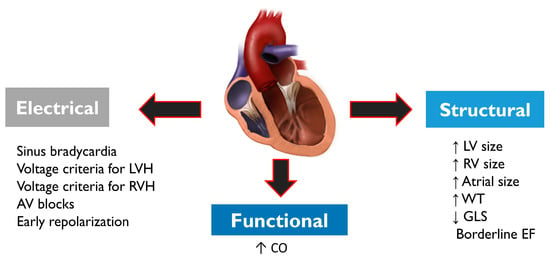
2.3. Role of cardiopulmonary resuscitation (CPR) and AEDs
References
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115.
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Association for European Cardiovascular Pathology. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows. Arch. 2017, 471, 691–705.
- Thiene, G.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Bruneval, P.; Buja, L.M.; Butany, J.; d’Amati, G.; de Gouveia, R.H.; et al. Association for European Cardiovascular Pathology and Society for Cardiovascular Pathology Task Force on Training in Cardiovascular Pathology. AECVP and SCVP 2009 recommendations for training in cardiovascular pathology. Cardiovasc. Pathol. 2010, 19, 129–135.
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963.
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980-2006. Circulation 2009, 119, 1085–1092.
- Eckart, R.E.; Shry, E.A.; Burke, A.P.; McNear, J.A.; Appel, D.A.; Castillo-Rojas, L.M.; Avedissian, L.; Pearse, L.A.; Potter, R.N.; Tremaine, L.; et al. Sudden death in young adults: An autopsy-based series of a population undergoing active surveillance. J. Am. Coll. Cardiol. 2011, 58, 1254–1261.
- Harmon, K.G.; Drezner, J.A.; Maleszewski, J.J.; Lopez-Anderson, M.; Owens, D.; Prutkin, J.M.; Asif, I.M.; Klossner, D.; Ackerman, M.J. Pathogeneses of sudden cardiac death in national collegiate athletic association athletes. Circ. Arrhythm. Electrophysiol. 2014, 7, 198–204.
- null
- Finocchiaro, G.; Sheikh, N.; Biagini, E.; Papadakis, M.; Maurizi, N.; Sinagra, G.; Pelliccia, A.; Rapezzi, C.; Sharma, S.; Olivotto, I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020, 17, 142–151.
- Behr, E.R.; Dalageorgou, C.; Christiansen, M.; Syrris, P.; Hughes, S.; Tome Esteban, M.T.; Rowland, E.; Jeffery, S.; McKenna, W.J. Sudden arrhythmic death syndrome: Familial evaluation identifies inheritable heart disease in the majority of families. Eur. Heart J. 2008, 29, 1670–1680.
- Papadakis, M.; Papatheodorou, E.; Mellor, G.; Raju, H.; Bastiaenen, R.; Wijeyeratne, Y.; Wasim, S.; Ensam, B.; Finocchiaro, G.; Gray, B.; et al. The Diagnostic Yield of Brugada Syndrome After Sudden Death With Normal Autopsy. J. Am. Coll. Cardiol. 2018, 71, 1204–1214.
- Lahrouchi, N.; Raju, H.; Lodder, E.M.; Papatheodorou, E.; Ware, J.S.; Papadakis, M.; Tadros, R.; Cole, D.; Skinner, J.R.; Crawford, J.; et al. Utility of Post-Mortem Genetic Testing in Cases of Sudden Arrhythmic Death Syndrome. J. Am. Coll. Cardiol. 2017, 69, 2134–2145.
- Gerull, B.; Brodehl, A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021, 18, 378–390.
- Finocchiaro, G.; Dhutia, H.; Gray, B.; Ensam, B.; Papatheodorou, S.; Miles, C.; Malhotra, A.; Fanton, Z.; Bulleros, P.; Homfray, T.; et al. Diagnostic yield of hypertrophic cardiomyopathy in first-degree relatives of decedents with idiopathic left ventricular hypertrophy. Europace 2020, 22, 632–642.
- Watson, C.J.; Stone, G.L.; Overbeek, D.L.; Chiba, T.; Burns, M.M. Performance-enhancing drugs and the Olympics. J. Intern Med. 2022, 291, 181–196.
- Adami, P.E.; Koutlianos, N.; Baggish, A.; Bermon, S.; Cavarretta, E.; Deligiannis, A.; Furlanello, F.; Kouidi, E.; Marques-Vidal, P.; Niebauer, J.; et al. Cardiovascular effects of doping substances, commonly prescribed medications and ergogenic aids in relation to sports: A position statement of the sport cardiology and exercise nucleus of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 559–575.
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297.
- Sawant, A.C.; Bhonsale, A.; te Riele, A.S.J.M.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H.; et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J. Am. Heart Assoc. 2014, 3, e001471.
- Finocchiaro, G.; Behr, E.R.; Tanzarella, G.; Papadakis, M.; Malhotra, A.; Dhutia, H.; Miles, C.; Diemberger, I.; Sharma, S.; Sheppard, M.N. Anomalous Coronary Artery Origin and Sudden Cardiac Death: Clinical and Pathological Insights From a National Pathology Registry. JACC Clin. Electrophysiol. 2019, 5, 516–522.
- Bagnall, R.D.; Weintraub, R.G.; Ingles, J.; Duflou, J.; Yeates, L.; Lam, L.; Davis, A.M.; Thompson, T.; Connell, V.; Wallace, J.; et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N. Engl. J Med. 2016, 374, 2441–2452.
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet Jean-Philippe Corrado, D.; Drezner, J.A.; Halle, M.; Hansen, D.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96.
- Corrado, D.; Pelliccia, A.; Bjørnstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; Panhuyzen-Goedkoop, N.; Deligiannis, A.; Solberg, E.; Dugmore, D.; et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: Proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2005, 26, 516–524.
- Maron, B.J.; Levine, B.D.; Washington, R.L.; Baggish, A.L.; Kovacs, R.J.; Maron, M.S. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 2: Preparticipation Screening for Cardiovascular Disease in Competitive Athletes: A Scientific Statement From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 2356–2361.
- Drezner, J.A.; O’Connor, F.G.; Harmon, K.G.; Fields, K.B.; Asplund, C.A.; Asif, I.M.; Price, D.E.; Dimeff, R.J.; Bernhardt, D.T.; Roberts, W.O. AMSSM Position Statement on Cardiovascular Preparticipation Screening in Athletes: Current evidence, knowledge gaps, recommendations and future directions. Br. J. Sports Med. 2017, 51, 153–167.
- American Academy of Family Physicians; American College of Sports Medicine; American Medical Society for Sports Medicine; American Orthopaedic Society for Sports Medicine; American Osteopathic Academy of Sports Medicine. Preparticipation Physical Evaluation, 4th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2012.
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 2006, 296, 1593–1601.
- Maron, B.J.; Haas, T.S.; Ahluwalia, A.; Rutten-Ramos, S.C. Incidence of cardiovascular sudden deaths in Minnesota high school athletes. Heart Rhythm 2013, 10, 374–377.
- Steinvil, A.; Chundadze, T.; Zeltser, D.; Rogowski, O.; Halkin, A.; Galily, Y.; Perluk, H.; Viskin, S. Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death: Proven fact or wishful thinking? J. Am. Coll. Cardiol. 2011, 57, 1291–1296.
- Vogiatzi, G.; Lazaros, G.; Oikonomou, E.; Lazarou, E.; Vavuranakis, E.; Tousoulis, D. Role of genetic testing in cardiomyopathies: A primer for cardiologists. World J. Cardiol. 2022, 14, 29–39.
- Magavern, E.F.; Finocchiaro, G.; Sharma, S.; Papadakis, M.; Borry, P. Time out: Ethical reflections on medical disqualification of athletes in the context of mandated pre-participation cardiac screening. Br. J. Sports Med. 2018, 52, 1207–1210.
- Malhotra, A.; Dhutia, H.; Finocchiaro, G.; Gati, S.; Beasley, I.; Clift, P.; Cowie, C.; Kenny, A.; Mayet, J.; Oxborough, D.; et al. Outcomes of Cardiac Screening in Adolescent Soccer Players. N. Engl. J. Med. 2018, 379, 524–534.
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453.
- Finocchiaro, G.; Dhutia, H.; D’Silva, A.; Malhotra, A.; Steriotis, A.; Millar, L.; Prakash, K.; Narain, R.; Papadakis, M.; Sharma, R.; et al. Effect of Sex and Sporting Discipline on LV Adaptation to Exercise. JACC Cardiovasc. Imaging 2017, 10, 965–972.
- Besenius, E.; Cabri, J.; Delagardelle, C.; Stammet, P.; Urhausen, A. Five Years-Results of a Nationwide Database on Sudden Cardiac Events in Sports Practice in Luxembourg. Dtsch Z. Sportmed. 2022, 73, 24–29.
- Drezner, J.A.; Rao, A.L.; Heistand, J.; Bloomingdale, M.K.; Harmon, K.G. Effectiveness of emergency response planning for sudden cardiac arrest in United States high schools with automated external defibrillators. Circulation 2009, 120, 518–525.
- Casa, D.J.; Almquist, J.; Anderson, S.A.; Baker, L.; Bergeron, M.F.; Biagioli, B.; Boden, B.; Brenner, J.S.; Carroll, M.; Colgate, B.; et al. The inter-association task force for preventing sudden death in secondary school athletics programs: Best-practices recommendations. J. Athl. Train. 2013, 48, 546–553.
- Harmon, K.G.; Asif, I.M.; Klossner, D.; Drezner, J.A. Incidence of sudden cardiac death in National Collegiate Athletic Association Athletes. Circulation 2011, 123, 1594–1600.
- Karam, N.; Pechmajou, L.; Narayanan, K.; Bougouin, W.; Sharifzadehgan, A.; Anys, S.; Weizman, O.; Perrot, D.; Waldmann, V.; Baganton, F.; et al. Evolutions of Incidence, Management, and Outcomes Over Time in Sports-Related Sudden Cardiac Arrest. J. Am. Coll Cardiol. 2022, 79, 238–246.




