1. Causes of Sudden Cardiac Death
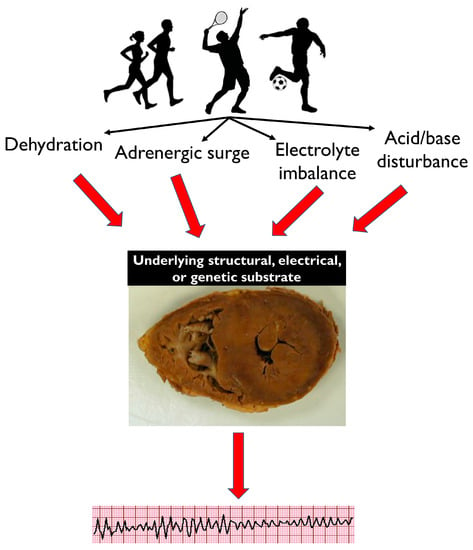
Common physiological effects of intense exercise, such as dehydration, adrenergic surge, electrolyte imbalance and acid/base disturbance, may not be well tolerated by athletes with a pathological electrical or structural substrate, resulting in potentially fatal arrhythmias (
Figure 1). A diverse spectrum of diseases is implicated in SCD, with variable prevalence dependent on the demographics of the victims and the circumstances of death. The majority of SCDs are attributable to atherosclerotic coronary artery disease and generally manifest in individuals in the fourth decade onward (
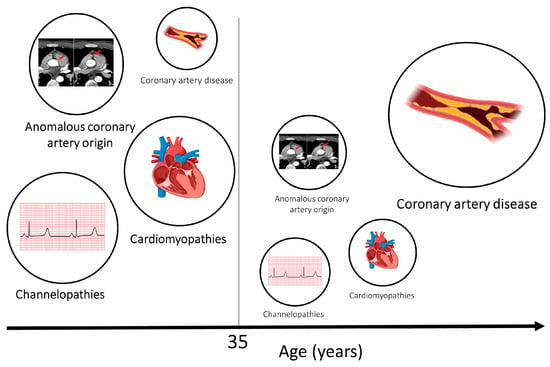
Figure 2). The primary cardiomyopathies and ion channelopathies are the predominant causes of SCD in the young (<35 years). The inherited nature of these conditions underscores the need for cardiac evaluation of first-degree relatives of the deceased. Post-mortem examination is an essential first diagnostic step to guiding clinical evaluation of surviving relatives toward inherited structural diseases or primary arrhythmogenic syndromes. Often, the post-mortem assessment is not performed by expert cardiac pathologists through standardized protocols, and this may result in inaccuracy in establishing the cause of death [
41,
42,
43].
Figure 1. Dehydration, adrenergic surge, electrolyte imbalance and acid/base disturbance are common physiological effects of intense exercise. While these are well tolerated by healthy athletes, they may cause potentially fatal arrhythmias in individuals with an underlying electrical, structural or genetic pathological cardiac substrate. This image of a heart at post-mortem is suggestive of HCM, and this is used as an example.
Figure 2. Aetiologies of SCD in athletes and age. Cardiomyopathies, channelopathies and congenital anomalies of the coronary arteries are prevalent causes in younger individuals. Atherosclerotic coronary artery disease is the most common cause of sudden cardiac death and sudden cardiac arrest in older individuals. The size of the circles relate to the relative frequency of SCD caused by the respective pathology. Age 35 has been used as a threshold as this is the most frequently used age in the literature; however, a clear line in terms of age is difficult to draw.
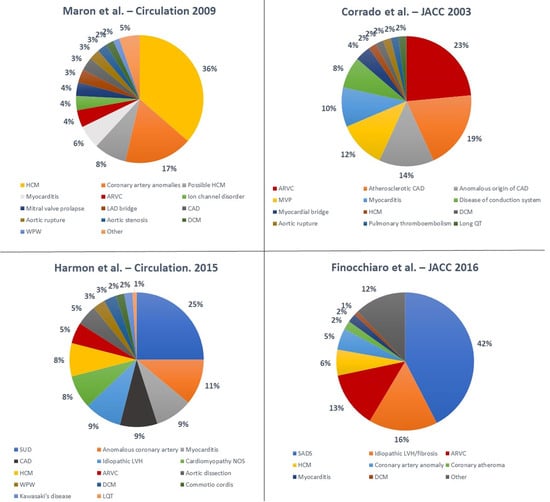
Numerous studies have been conducted to elucidate the underlying aetiologies of SCD in athletes (
Figure 3) [
18,
21,
41,
44,
45]. The variability in terms of results is significant. Hypertrophic cardiomyopathy (HCM) is traditionally considered the most common cause of SCD in young athletes in the United States; Maron et al. [
21] reported on the National Registry of SCD in Athletes (based in the United States) and showed that HCM accounted for 36% of all SCD events in young athletes. These data are based on a large cohort but are limited by the fact that an autopsy was not performed in all athletes and was carried out by an expert cardiac pathologist only in the minority of cases.
Figure 3. Summary of main studies describing the causes of SCD in athletes. Abbreviations: ARVC—arrhythmogenic right ventricular cardiomyopathy; CAD—coronary artery disease; DCM—dilated cardiomyopathy; HCM—hypertrophic cardiomyopathy; LAD—left anterior descending artery; LQT—long QT; LVH—left ventricular hypertrophy; MVP—mitral valve prolapse; NOS—not otherwise specified; SADS—sudden arrhythmic death syndrome; SUD—sudden unexplained death; WPW—Wolff–Parkinson–White [
18,
21,
41,
45].
Recent studies have reported different results. A study by Corrado et al. [
18] showed that in the Veneto region in Italy, arrhythmogenic right ventricular cardiomyopathy (ARVC) was the most common cause of SCD in young athletes (23% of cases), while HCM accounted for only 2% of deaths. Mandatory pre-participation screening (using the ECG) in Italy may explain the reported low incidence of HCM. In fact, ECG abnormalities are common in HCM, and the disqualification of affected athletes may have prevented SCDs [
46].
Eckart et al. [
44] examined 902 cases of SCD in active military personnel from the Department of Defense in the United States. These individuals were in active service, and therefore were required to maintain a certain level of fitness. In young individuals (<35 years), the heart appeared structurally normal at the post-mortem examination in 41% of cases. Hypertrophic cardiomyopathy accounted for only 13% of cases. In older individuals the most common cause of death was atherosclerotic disease (73%). This study was retrospective, and most of autopsies were conducted by a local medical examiner with no regular involvement of a specialist cardiac pathologist. Harmon et al. [
45] reported that a structurally normal heart at the post-mortem examination was the most common finding (25%) in 64 cases of college athletes who died suddenly. Coronary artery anomalies were the second most frequent cause (11%), and HCM accounted for 8% of the cases.
Finocchiaro et al. [
41] described a cohort of 357 athletes who died suddenly in the United Kingdom where the post-mortem examination was performed by an expert cardiac pathologist. The most common finding at the post-mortem examination was a normal heart (42% of cases), followed by myocardial disease including idiopathic left ventricular hypertrophy (LVH) and idiopathic fibrosis (16%), ARVC (13%), and HCM (6%). Coronary artery anomalies were found in 5% of cases.
The interpretation of the post-mortem results is a complex task and uncertainty may exist about the exact significance of certain pathological findings and their causal relationship with SCD. The clinical significance of a structurally normal heart with normal toxicology (defined as sudden arrhythmic death syndrome (SADS)) in the context of SCD is not fully understood. In SADS cases, death is most likely caused by primary arrhythmia syndromes, such as long QT syndrome, Brugada syndrome or catecholaminergic polymorphic tachycardia. Genetic testing of the deceased proband (molecular autopsy) and family screening of family members may help in providing a unifying diagnosis. Recent studies showed that up to 50% of families of SADS victims are affected by an inherited cardiac condition (usually a channelopathy) that can be linked with the SCD in the proband [
47,
48,
49]. In some cases of SADS or unexplained cardiac arrest, pathogenic variants in cardiomyopathy-related genes are found, which raises the possibility of an arrhythmic phenotype preceding a fully expressed structural phenotype [
50].
Idiopathic fibrosis and idiopathic LVH are common autopsy findings in young athletes who have died suddenly. The clinical significance of these entities is unclear as they may be incidental and innocent bystanders or may constitute substrates for potentially fatal arrhythmias. Finocchiaro et al. [
51] investigated whether idiopathic LVH and familial HCM are part of the same disease. These authors comprehensively assessed first-degree family members of 46 decedents with idiopathic LVH and found that none fulfilled diagnostic criteria for HCM, suggesting that idiopathic LVH is a distinct disease entity.
Although genetic and inherited cardiac conditions are the predominant causes of SCD in young individuals, the contribution of non-genetic and “acquired” factors may be relevant. Specifically, drugs, alcohol and smoking may act as second hits in predisposed individuals, resulting in maladaptation and potentially fatal arrhythmias. The use of performance-enhancing drugs has progressively increased recently. These drugs are constantly under review by the World Anti-Doping Agency (WADA) [
52]. Side effects are many and depend on the type of substance, the amount and the duration of use, leading in some cases to tragic consequences, including SCD [
53].
Circumstances of Death
Sudden cardiac death in athletes often occurs during exercise, but it can also occur at rest and sometimes during sleep. A recent study on athletes in the United Kingdom showed that 61% of athletes died suddenly during exertion, including a small proportion of individuals (4%) who died during altercation. Of the individuals who died at rest, one-third died while sleeping [
41]. Certain cardiac conditions, such as arrhythmogenic cardiomyopathy (AC) and coronary artery anomalies, often lead to SCD during exertion [
41]. Intense exercise has been shown to be particularly deleterious in AC, where the higher risk of fatal arrhythmias is often accompanied by a worsening of the phenotype [
54,
55]. Coronary artery anomalies comprise many anatomical subtypes. A retrospective analysis of 30 cases with an anomalous origin of the coronary artery revealed that anomalous left coronary artery arising from the right sinus of Valsalva was mostly associated with SCD during exercise (73% of SCDs occurred during exercise, compared to 18% in the anomalous right coronary artery arising from the left sinus of Valsalva) [
56]. In contrast, in cases of SADS, SCD occurs more often at rest or during sleep [
57].
2. Prevention of SCD in Athletes
Sudden cardiac death in athletes may be prevented through the implementation of policies aimed at identifying cardiac conditions that may pose a risk in asymptomatic individuals (screening) and policies that increase the likelihood of successful resuscitation of cardiac arrests.
2.1. Pre-Participation Cardiac Screening
Both the American Heart Association/American College of Cardiology (AHA/ACC) and the European Society of Cardiology (ESC) recommend pre-participation cardiac screening with the aim of identifying cardiac conditions that pose a risk of SCD [
58,
59,
60,
61,
62]. There is no global consensus on whether or how cardiac screening should be performed, with high variability among countries, sports governing bodies and level of competition. Both American and European guidelines suggest that medical history and physical examination should be part of the cardiac screening assessment [
57,
58,
59,
60,
61]. However, while the 12-lead ECG is the key investigation proposed by the ESC, this test is not recommended by the AHA, which instead focuses on personal and family history and physical examination [
58,
59,
60]. Corrado et al. [
16] showed that the implementation of a mandatory pre-participation cardiac screening program with the use of the ECG led to a significant decrease in the incidence of SCD in athletes. In the Veneto region of Italy, the annual incidence of SCD in athletes decreased by 89% after cardiac screening become compulsory in 1982. In contrast, Maron et al. [
63] analysed 13 cases of SCD that occurred in high school student athletes in Minnesota over a 26-year period and reported that only 4 (31%) individuals had cardiovascular conditions that could have been reliably detected through cardiac screening with history, examination and ECG. Furthermore, Steinvil et al. [
64] showed that the effect of implementing mandatory pre-participation cardiac screening with ECG and exercise testing in Israel in 1997 did not lead to a significant change in documented events of SCD in competitive athletes. This study relied on the systematic search of two main newspapers in Israel, which constitutes a significant limitation as data were not collected through a prospective database, resulting in a possible underestimation of the events.
If initial tests are suspicious for cardiac disease, further investigations, such as echocardiogram, cardiovascular magnetic resonance, cardio-pulmonary exercise testing and family testing, are recommended. In the presence of a suspicious phenotype, genetic testing may be helpful for diagnosis and risk stratification [
58]. Although specific genetic testing can be helpful, in the absence of a suspicious phenotype or a positive proband, genetic screening is not routinely carried out [
65]. This is because it has a low yield and variants of unknown significance, which may not be clinically significant, may be found [
65].
While pre-participation screening may be ethically justified to prevent SCD in athletes, there are a number of complex ethical issues relating to disqualification decisions [
66]. Athletes, especially those competing at very high levels, may perceive disqualification based on the results generated by screening as discrimination on the basis of a medical condition. Moreover, disqualification can cause significant psychological stress in athletes, which may affect their well-being in the long term.
A shared decision-making process regarding sport participation is always advisable, taking into account the lack of robust evidence on the risk of exercise in individuals with cardiac disease. There are certain conditions and situations wherein exercise appears to be deleterious and carries significant risk. These include AC prior cardiac arrest or unexplained syncope, symptomatic/obstructive HCM, DCM with significant impairment of systolic function and/or high-risk genotypes (lamin A/C—filamin C) [
58].
The use of mass cardiac screening in athletes remains controversial. The most cited issues are those relating to the economic sustainability of a nationwide screening program, the uncertain benefits in terms of SCD reduction, the potential for false positives and consequential unnecessary disqualifications and the fact that screening does not prevent all cardiac deaths among young athletes [
38,
61].
2.2. Differential Diagnosis between “Athlete’s Heart” and Pathological Cardiac Conditions
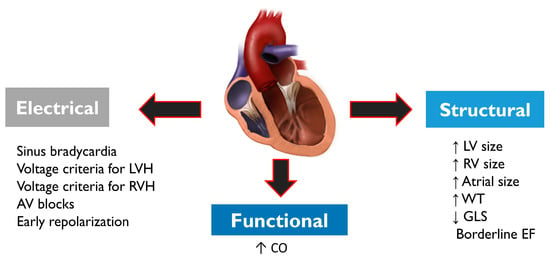
Pre-participation cardiac screening in athletes may lead to further investigations aimed at ruling out cardiac disease. Diagnosis is often complex as athletes usually exhibit a series of electrical, structural and functional physiological changes (
Figure 4), which may overlap with cardiac pathology. Structural changes on echocardiogram include an increased cardiac chamber size and myocardial wall thickness. Electrical changes on ECG include sinus bradycardia or arrhythmia, first-degree or Mobitz type 1 atrioventricular block, voltage criteria for ventricular hypertrophy, incomplete right bundle branch block, T-wave inversion and J-point elevation with ascending ST segments [
67]. Functional changes include increased diastolic filling and stroke volume. Demographic factors such as sex and ethnicity may influence cardiac adaptation to exercise in athletes; concentric remodelling and hypertrophy of the left ventricle are more prevalent in males, while females more frequently exhibit eccentric LV hypertrophy [
32].
Figure 4. Structural, electrical and functional physiological changes commonly observed in athletes. Physiological changes may overlap with cardiac conditions that may pose a risk of SCD, and therefore differential diagnosis is very important. Abbreviations: AV—atrioventricular; CO—cardiac output; EF—ejection fraction; GLS—global longitudinal strain; LV—left ventricle; LVH—left ventricular hypertrophy; RV—right ventricle; RVH—right ventricular hypertrophy; WT: wall thickness.
2.3. Role of cardiopulmonary resuscitation (CPR) and AEDs
A cornerstone in the prevention of SCD in athletes is the immediate availability of quality CPR performed by bystanders and AEDs. A study based on a dedicated Luxembourg nationwide database showed that the ratio of survival among patients receiving bystander CPR during a cardiac arrest was about 50% [
17]. In contrast, all cases of cardiac arrests not having CPR were fatal. The greatest determinant of survival after SCA is the time from collapse to defibrillation, with survival rates declining from 7% to 10% per minute for every minute lost [
19].
AEDs should be promptly available so that a first shock can be applied within 3 minutes of the collapse [
68]. The role of education in the general population is fundamental, as well as the deployment of external defibrillators in public areas and training and sports grounds to exponentially increase the chance of better outcomes [
20]. A recent investigation by Karam et al. [
69] described a substantial stability of incidence of sport-related SCA between 2005 and 2018; in the first 2-year period of the study, the estimated incidence was 7.00 per million inhabitants/years compared to 6.24 per million inhabitants/years in the last 2 years. The increased education of the general population in terms of bystander cardiopulmonary resuscitation (CPR) and use of public automated external defibrillators (AEDs) led to a significant improvement in survival to hospital discharge rates (23.8% in the first period compared to 66.7% in the last) [
69].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd10020068