Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jin Joo | -- | 1384 | 2023-05-16 06:52:30 | | | |
| 2 | Conner Chen | Meta information modification | 1384 | 2023-05-17 05:17:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Joo, J.; Jeong, J.; Park, H.J. Blood Inflammatory Biomarkers in Parkinson’s Disease Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/44343 (accessed on 07 February 2026).
Joo J, Jeong J, Park HJ. Blood Inflammatory Biomarkers in Parkinson’s Disease Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/44343. Accessed February 07, 2026.
Joo, Jin, Jongmin Jeong, Hue Jung Park. "Blood Inflammatory Biomarkers in Parkinson’s Disease Patients" Encyclopedia, https://encyclopedia.pub/entry/44343 (accessed February 07, 2026).
Joo, J., Jeong, J., & Park, H.J. (2023, May 16). Blood Inflammatory Biomarkers in Parkinson’s Disease Patients. In Encyclopedia. https://encyclopedia.pub/entry/44343
Joo, Jin, et al. "Blood Inflammatory Biomarkers in Parkinson’s Disease Patients." Encyclopedia. Web. 16 May, 2023.
Copy Citation
Parkinson’s disease (PD) is the second most common inflammatory neurodegenerative disorder after dementia. Preclinical and epidemiological data strongly suggest that chronic neuroinflammation slowly induces neuronal dysfunction. Activated microglia secrete several neurotoxic substances, such as chemokines and proinflammatory cytokines, which may promote blood–brain barrier (BBB) permeabilization.
Parkinson’s disease
inflammatory biomarkers
surgery
1. Introduction
Parkinson’s disease (PD) is the second most common inflammatory neurodegenerative disorder after dementia, affecting 7–10 million people worldwide [1]. About 2–3% of elderly patients (aged ≥ 65 years) are affected by PD [2]. People with PD suffer motor symptoms such as bradykinesia, rigidity, resting tremor, and postural instability. In addition, patients often complain of ‘non-motor symptoms’ such as cognitive impairment, anxiety, depression, hypothermia, constipation, bowel and rapid eye movement sleep behavior disorder, and autonomic nervous system disorders [2][3].
PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta, the appearance of Lewy bodies (intracellular inclusions of aggregated a-synuclein), and the presence of neuroinflammation [4][5][6][7]. Although the mechanism of neuron loss in PD is unclear, inflammation and the peripheral immune system play key roles [7][8][9]. Preclinical and epidemiological data suggest that chronic neuroinflammation induces neuronal dysfunction during the asymptomatic stage of PD [6][10]. The activation of resident microglia precedes dopamine neuron loss [11][12]. Activated microglia secrete several neurotoxic substances, such as chemokines and proinflammatory cytokines, which may cause blood–brain barrier (BBB) permeabilization and subsequent infiltration of peripheral leukocytes into the central nervous system (CNS) [11][12][13].
There is currently no cure for PD to prevent PD or delay its progression, mainly due to the still limited comprehension of the events ultimately leading to neurodegeneration. Available treatments for PD are only symptomatic, aimed at relieving the loss of brain dopaminergic neurons by using levodopa (the dopamine precursor), some dopaminergic agonists, and other indirect dopaminergic agents. Surgery, including deep brain stimulation, may be considered in advanced PD patients who fail to respond to levodopa [14].
CD4+ T lymphocytes play a pivotal role in orchestrating immune responses implicated not only in the pathogenesis of inflammatory diseases, but also in host defense. CD4+ T cells include proinflammatory cells such as T helper (Th) 1 and Th17, and anti-inflammatory cells such as Th2 and T regulatory cells (Tregs) [15][16]. Interestingly, both animal models of PD and clinical studies suggest that Th1 and Th17 can be detrimental to dopamine neurons, whereas Th2 and Tregs are neuroprotective [17][18]. Indeed, the number of circulating CD4+ T cells is reduced in patients with PD [19], but the relative proportions and functional profiles of subdivided cell populations are controversial. The decrease in CD4+ T cells seen in the peripheral blood of patients with PD is mainly due to decreases in Th2, Th17, and Tregs [20][21]. As a result, Th1 T cells, the absolute numbers of which are similar to healthy controls, increase in PD patients compared to other T cells, resulting in a Th1 bias. Consequently, the production of IFN-γ and TNF-α by Th1 cell lineages increases [21]. The results of studies on the serum levels of cytokines such as IFN-γ and TNF-α secreted by Th1 T cells, IL-8 and IL-10 secreted by Th2 T cells, and IL-17 secreted by Th17 in PD patients are not uniform [19][22][23][24][25][26][27]. In addition, the relationships between serum cytokine levels and motor and non-motor symptoms of PD are controversial [19][23][26][27].
Surgical stress and anesthesia induce inflammatory responses by disturbing the balance between pro- and anti-inflammatory cytokines [28], which may exacerbate the neuroinflammatory response in PD patients. The effects of inhalational anesthetics on the inflammatory response are controversial [29][30][31][32]. Meanwhile, Shan et al. [33] reported that sevoflurane worsened the prognosis of PD in a Drosophila model. There are few studies on the effect of the immune response on PD symptoms and prognosis after surgery and anesthesia [34][35][36][37].
2. Blood Inflammatory Biomarkers in PD Patients
Only seven markers (CRP, IL-1β, IL-2, IL-6, IL-8, IFN-γ, TNF-α) were reported in more than 5 of 51 studies. The most frequently studied inflammatory biomarkers are CRP and IL-1β [26][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58]. These two biomarkers show consistently higher levels in PD patients than healthy controls. Data for IL-2, IL-6, IFN-γ, and TNF-α are controversial. Some studies report higher blood levels of these biomarkers, while others found no differences between PD patients and healthy controls, or lower levels in the former group [27][38][41][42][43][44][49][50][51][53][54][55][56][59][60][61][62][63][64][65][66][67][68][69][70][71][72]. Blood markers evaluated to date are listed in Table 1 and Figure 1.
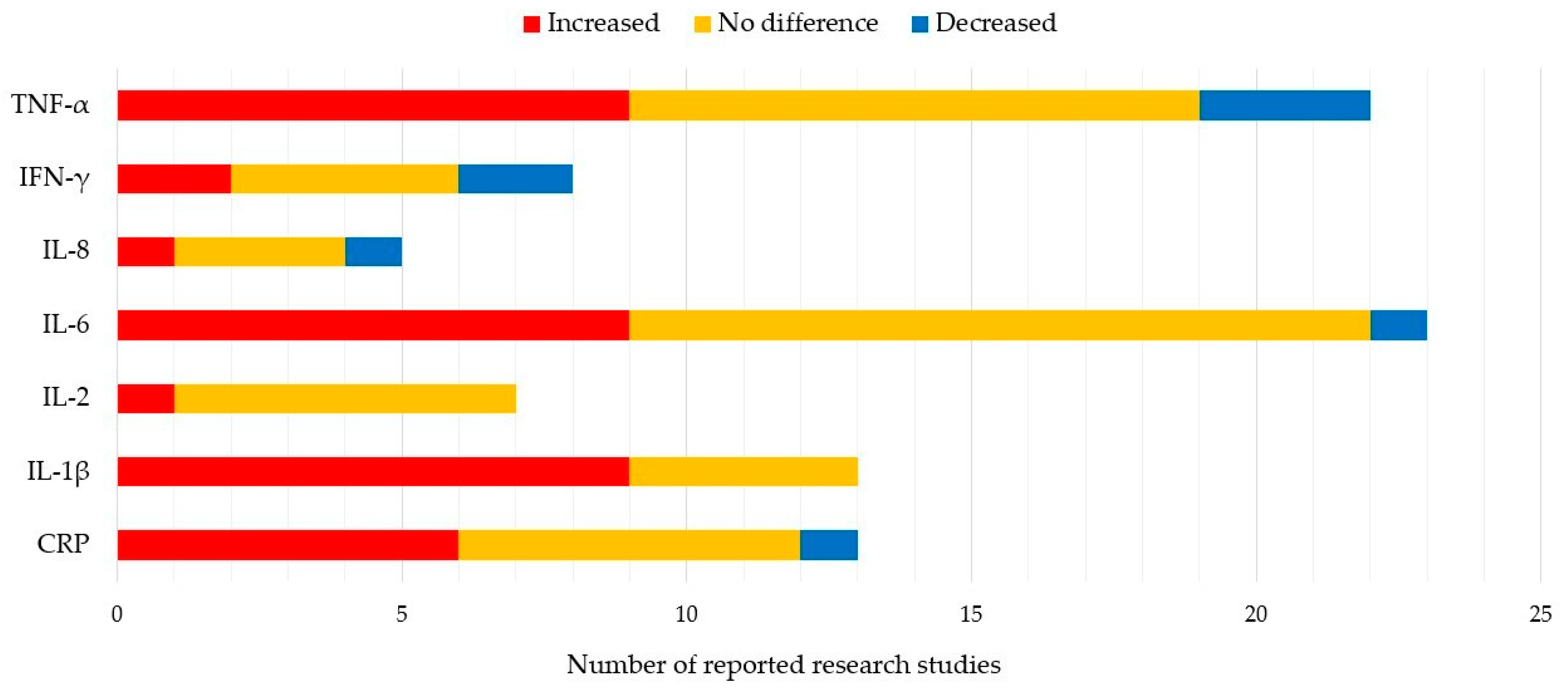
Figure 1. 7 Most reported blood biomarkers and their value relative to healthy controls.
Table 1. Blood biomarkers and their related symptoms.
| Clinical Trials | Blood Markers | Related Symptoms |
|---|---|---|
| Alrafiah et al. [55] | IL-1β, IL-6, TNF-α | |
| Andican et al. [39] | CRP, ICAM-1 | |
| Bagheri et al. [73] | CXCL 12, CXCR4 | |
| Baran et al. [46] | CRP, HMGB1 | |
| Brockmann et al. [52] | FABP, IL-10, IL-12p40, SCF, BDNF | |
| Calvani et al. [65] | MIP-1α, MIP-1β, IL-8, IL-9 | |
| Carvalho et al. [74] | S100B * | nonmotor |
| Chatterjee et al. [57] | IL-1β, NLRP3 | |
| Csencsits-Smith et al. [71] | MCP-1, IP-10, TNF-α | |
| Delgado-Alvarado et al. [62] | IL-6 * | motor, nonmotor |
| Dommershuijsen et al. [48] | CRP | |
| Dufek et al. [75] | IL-6 * | mortality |
| Dumitrescu et al. [76] | calprotectin | |
| Eidson et al. [69] | IL-8 *, IFN-γ, NGAL, TNF-α | motor, nonmotor |
| Fan et al. [58] | IL-1β *, NLRP3 | motor, nonmotor |
| Green et al. [77] | IL-6 *, IL-17A *, TNF-α, TGF-β | motor, nonmotor |
| Gupta et al. [78] | Fractakine *, 3-NT * | motor |
| Gupta et al. [27] | IL-8, TNF- α | |
| Herlofson et al. [79] | IL1-Ra *, VCAM-1 * | nonmotor |
| Hu et al. [50] | IL-1β, TNF-α | |
| Jin et al. [47] | CRP | |
| Karpenko et al. [53] | IL-1β, IL-1Ra, IL-6, IL-10 *, TNF-α * | nonmotor |
| Kim et al. [43] | CRP, IL-1β, IL-2, IL-6, IL-10 *, TNF-α | nonmotor |
| King et al. [44] | CRP, IL-2 *, IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α | motor |
| Kouchaki et al. [72] | IL-27 *, TNF-α * | motor |
| Koziorowski et al. [49] | IL-1β, IL-6 *, IL-10, IL-12, TNF-α, NT-proCNP | motor |
| Kwiatek-Majkusiak et al. [63] | IL-6 | |
| Lerche et al. [80] | FABP *, TNF-α *, CA-125 *, BDNF* | motor, nonmotor |
| Lian et al. [56] | IL-1β, IL-6 * | motor |
| Lin et al. [66] | IL-1β, IL-2, IL-4, IL-6, IL-13, IL-18, IFN-γ, TNF-α | |
| Lindqvist et al. [26] | CRP, IL-6 *, sIL-2R, TNF-a | nonmotor |
| Mahlknecht et al. [59] | MCP-4, ICAM-1, IL-2, IL-6, Leptin, PDGF-BB, prolactin, RANTES | |
| Martin-Ruiz et al. [70] | CRP *, IL-6 *, IL-10, TNF-α | nonmotor |
| Miliukhina et al. [64] | MCP-1, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-21, IL-23, INF-γ, TNF-α | |
| Milyukhina et al. [51] | IL-1β *, IL-6, IL-10 *, TNF-α * | nonmotor |
| Pereira et al. [81] | IL-6 * | nonmotor |
| Perner et al. [82] | VCAM-1 * | motor |
| Rathnayake et al. [67] | IL-10, IFN-γ, TNF-α | |
| Rocha et al. [54] | IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNF-α | |
| Rocha et al. [83] | sTNFR1 *, sTNFR2 * | nonmotor |
| Roy et al. [84] | NLRP3 | |
| Santos-Garcia et al. [45] | CRP* | motor |
| Sawada et al. [85] | CRP* | nonmotor |
| Schroder et al. [60] | IL-2, IL-4, IL-5, IL-6, IL-9, IL10, IL-13, IL-17A, IL-17F, IL-21, IL-22, IFN-γ, TNF-α, 1111, CCL17, CCL20, CXCL1, CXCL5, CXCL9, CXCL11, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, RANTES |
|
| Sun et al. [86] | C3 *, C4 | nonmotor |
| Tang et al. [61] | RANTES *, IL-6 * | motor |
| Ton et al. [38] | CRP, IL-6 | |
| Umemura et al. [87] | CRP * | motor |
| Usenko et al. [68] | MCP-1, IL-4, IL-6, IL-10*, INF-γ, TNF-α * | nonmotor |
| Vesely et al. [23] | C3 *, C4*, IL-6 * | nonmotor |
| Vesely et al. [88] | C3 * | nonmotor |
| Wang et al. [42] | CRP *, IL-1β, sIL-2R *, IL-6, IFN-γ, TNF-α* | nonmotor |
| Yang et al. [89] | IL-6, IL-10, IL-17, IL-23, TGF-β | |
| Yilmaz et al. [90] | IL-12p40 * | motor, nonmotor |
BDNF, brain-derived neurotrophic factor; C, complement; CA-125, cancer antigen 125; CCL, C-C motif chemokine; CRP, C-reactive protein; CXCL 12, C-X-C motif chemokine ligand 12; CXCR4, C-X-C chemokine receptor type 4; FABP, fatty acid-binding protein; HMGB1, high-mobility group box 1 protein; ICAM-1, intercellular adhesion molecule 1; IL1-Ra, interleukin-1 receptor antagonist; IP-10, interferon gamma-induced protein 10; IFN-γ, interferon gamma; MCP, monocyte chemoattractant protein-1; 3-NT, 3-nitrotyrosine; MIP, macrophage inflammatory protein; NGAL, neutrophil gelatinase associated lipocalin; NLRP3, NLR family pyrin domain containing 3; NT-proCNP, N-terminal pro c-type natriuretic peptide; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated upon activation, normal T-cell expressed and presumably secreted; S100B, S100 calcium binding protein B; SCF, stem cell factor; sIL-2R, soluble interleukin-2 receptors; sTNFR, soluble tumor necrosis factor receptors; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor alpha; VCAM-1, vascular cell adhesion protein 1; red colored, increased in Parkinson’s disease patients relative to healthy controls; blue colored, decreased in Parkinson’s disease patients relative to healthy controls; green colored, positively correlated with symptom; orange colored, negatively correlated with the symptom; *, significantly correlated with the symptom.
Although many studies have focused on proinflammatory profiles, inflammation is a balance between pro- and anti-inflammatory processes. CD4+ T lymphocytes orchestrate an effective immune response during host defense, as well as in the pathogenesis of inflammatory diseases. CD4+ T cells can select for proinflammatory phenotypes such as Th 1 and Th17 cells, as well as anti-inflammatory phenotypes such as Th2 and Tregs [15][16]. Results from animal models of PD and clinical studies suggest that Th1 and Th17 cells are detrimental to neurons, while Th2 and Tregs are neuroprotective [17][18].
References
- Delenclos, M.; Jones, D.R.; McLean, P.J.; Uitti, R.J. Biomarkers in Parkinson’s disease: Advances and strategies. Parkinsonism Relat. Disord. 2016, 22, S106–S110.
- Chaudhuri, K.R.; Odin, P.; Antonini, A.; Martinez-Martin, P. Parkinson’s disease: The non-motor issues. Parkinsonism Relat. Disord. 2011, 17, 717–723.
- Corrado, L.; De Marchi, F.; Tunesi, S.; Oggioni, G.D.; Carecchio, M.; Magistrelli, L.; Tesei, S.; Riboldazzi, G.; Di Fonzo, A.; Locci, C.; et al. The Length of SNCA Rep1 Microsatellite May Influence Cognitive Evolution in Parkinson’s Disease. Front. Neurol. 2018, 9, 213.
- Eriksen, J.L.; Wszolek, Z.; Petrucelli, L. Molecular pathogenesis of Parkinson disease. Arch. Neurol. 2005, 62, 353–357.
- Schapira, A.H.; Jenner, P. Etiology and pathogenesis of Parkinson’s disease. Mov. Disord. 2011, 26, 1049–1055.
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.Y.; Choi, D.K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm. 2013, 2013, 952375.
- McGeer, P.L.; McGeer, E.G. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat. Disord. 2004, 10, S3–S7.
- González, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274, 1–13.
- Mosley, R.L.; Hutter-Saunders, J.A.; Stone, D.K.; Gendelman, H.E. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009381.
- Lee, J.K.; Tran, T.; Tansey, M.G. Neuroinflammation in Parkinson’s disease. J. Neuroimmun. Pharmacol. 2009, 4, 419–429.
- Monahan, A.J.; Warren, M.; Carvey, P.M. Neuroinflammation and peripheral immune infiltration in Parkinson’s disease: An autoimmune hypothesis. Cell Transpl. 2008, 17, 363–372.
- McGeer, P.L.; Itagaki, S.; Akiyama, H.; McGeer, E.G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. 1988, 24, 574–576.
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin. Neurosci. Res. 2006, 6, 261–281.
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612.
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Ann. Rev. Immunol. 2010, 28, 445–489.
- O’Shea, J.J.; Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010, 327, 1098–1102.
- González, H.; Contreras, F.; Pacheco, R. Regulation of the Neurodegenerative Process Associated to Parkinson’s Disease by CD4+ T-cells. J. Neuroimmun. Pharmacol. 2015, 10, 561–575.
- Tahmasebinia, F.; Pourgholaminejad, A. The role of Th17 cells in auto-inflammatory neurological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79 (Pt B), 408–416.
- Jiang, S.; Gao, H.; Luo, Q.; Wang, P.; Yang, X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 1373–1380.
- Kustrimovic, N.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Comi, C.; Mauri, M.; Minafra, B.; Riboldazzi, G.; et al. Dopaminergic Receptors on CD4+ T Naive and Memory Lymphocytes Correlate with Motor Impairment in Patients with Parkinson’s Disease. Sci. Rep. 2016, 6, 33738.
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J. Neuroinflamm. 2018, 15, 205.
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003.
- Veselý, B.; Dufek, M.; Thon, V.; Brozman, M.; Királová, S.; Halászová, T.; Koriťáková, E.; Rektor, I. Interleukin 6 and complement serum level study in Parkinson’s disease. J. Neural. Transm (Vienna) 2018, 125, 875–881.
- Schlachetzki, J.C.; Winkler, J. The innate immune system in Parkinson’s disease: A novel target promoting endogenous neuroregeneration. Neural Regen. Res. 2015, 10, 704–706.
- Storelli, E.; Cassina, N.; Rasini, E.; Marino, F.; Cosentino, M. Do Th17 Lymphocytes and IL-17 Contribute to Parkinson’s Disease? A Systematic Review of Available Evidence. Front. Neurol. 2019, 10, 13.
- Lindqvist, D.; Kaufman, E.; Brundin, L.; Hall, S.; Surova, Y.; Hansson, O. Non-motor symptoms in patients with Parkinson’s disease-correlations with inflammatory cytokines in serum. PLoS ONE 2012, 7, e47387.
- Gupta, V.; Garg, R.K.; Khattri, S. Levels of IL-8 and TNF-α decrease in Parkinson’s disease. Neurol. Res. 2016, 38, 98–102.
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768.
- Pirbudak Cocelli, L.; Ugur, M.G.; Karadasli, H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr. Ther. Res. Clin. Exp. 2012, 73, 41–51.
- Jiao, B.; Yang, C.; Huang, N.N.; Yang, N.; Wei, J.; Xu, H. Relationship between Volatile Anesthetics and Tumor Progression: Unveiling the Mystery. Curr. Med. Sci. 2018, 38, 962–967.
- Marana, E.; Russo, A.; Colicci, S.; Polidori, L.; Bevilacqua, F.; Viviani, D.; Di Stasio, E. Desflurane versus sevoflurane: A comparison on stress response. Minerva Anestesiol. 2013, 79, 7–14.
- Allaouchiche, B.; Debon, R.; Goudable, J.; Chassard, D.; Duflo, F. Oxidative stress status during exposure to propofol, sevoflurane and desflurane. Anesth. Analg. 2001, 93, 981–985.
- Shan, Z.; Cai, S.; Zhang, T.; Kuang, L.; Wang, Q.; Xiu, H.; Wen, J.; Gu, H.; Xu, K. Effects of sevoflurane on leucine-rich repeat kinase 2-associated Drosophila model of Parkinson’s disease. Mol. Med. Rep. 2015, 11, 2062–2070.
- Goh, G.S.; Zeng, G.J.; Tay, D.K.; Lo, N.N.; Yeo, S.J.; Liow, M.H.L. Patients With Parkinson’s Disease Have Poorer Function and More Flexion Contractures After Total Knee Arthroplasty. J. Arthropl. 2021, 36, 2325–2330.
- Oğuz, E.; Öztürk, İ.; Özkan, D.; Ergil, J.; Aydın, G.B. Parkinson’s Disease and Spinal Anaesthesia. Turk. J. Anaesthesiol. Reanim. 2014, 42, 280–282.
- Rodríguez-Merchán, E.C.; Kalbakdij-Sánchez, C. The impact of Parkinson’s disease on results of primary total knee arthroplasty. EFORT Open Rev. 2022, 7, 701–709.
- Rondon, A.J.; Tan, T.L.; Schlitt, P.K.; Greenky, M.R.; Phillips, J.L.; Purtill, J.J. Total Joint Arthroplasty in Patients With Parkinson’s Disease: Survivorship, Outcomes, and Reasons for Failure. J. Arthropl. 2018, 33, 1028–1032.
- Ton, T.G.; Jain, S.; Biggs, M.L.; Thacker, E.L.; Strotmeyer, E.S.; Boudreau, R.; Newman, A.B.; Longstreth, W.T., Jr.; Checkoway, H. Markers of inflammation in prevalent and incident Parkinson’s disease in the Cardiovascular Health Study. Parkinson. Relat. Disord. 2012, 18, 274–278.
- Andican, G.; Konukoglu, D.; Bozluolcay, M.; Bayülkem, K.; Firtiına, S.; Burcak, G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol. Belg. 2012, 112, 155–159.
- Sawada, H.; Oeda, T.; Umemura, A.; Tomita, S.; Hayashi, R.; Kohsaka, M.; Yamamoto, K.; Sudoh, S.; Sugiyama, H. Subclinical elevation of plasma C-reactive protein and illusions/hallucinations in subjects with Parkinson’s disease: Case-control study. PLoS ONE 2014, 9, e85886.
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016, 73, 1316–1324.
- Wang, X.M.; Zhang, Y.G.; Li, A.L.; Long, Z.H.; Wang, D.; Li, X.X.; Xia, J.H.; Luo, S.Y.; Shan, Y.H. Relationship between levels of inflammatory cytokines in the peripheral blood and the severity of depression and anxiety in patients with Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3853–3856.
- Kim, R.; Kim, H.J.; Kim, A.; Jang, M.; Kim, A.; Kim, Y.; Yoo, D.; Im, J.H.; Choi, J.H.; Jeon, B. Peripheral blood inflammatory markers in early Parkinson’s disease. J. Clin. Neurosci. 2018, 58, 30–33.
- King, E.; O’Brien, J.; Donaghy, P.; Williams-Gray, C.H.; Lawson, R.A.; Morris, C.M.; Barnett, N.; Olsen, K.; Martin-Ruiz, C.; Burn, D.; et al. Inflammation in mild cognitive impairment due to Parkinson’s disease, Lewy body disease, and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2019, 34, 1244–1250.
- Santos-García, D.; de Deus Fonticoba, T.; Suárez Castro, E.; Aneiros Díaz, A.; Paz González, J.M.; Feal Panceiras, M.J.; García Sancho, C.; Jesús, S.; Mir, P.; Aguilar, M.; et al. High ultrasensitive serum C-reactive protein may be related to freezing of gait in Parkinson’s disease patients. J. Neural. Transm. 2019, 126, 1599–1608.
- Baran, A.; Bulut, M.; Kaya, M.C.; Demirpençe, Ö.; Sevim, B.; Akıl, E.; Varol, S. High-sensitivity C-reactive protein and high mobility group box-1 levels in Parkinson’s disease. Neurol. Sci. 2019, 40, 167–173.
- Jin, H.; Gu, H.Y.; Mao, C.J.; Chen, J.; Liu, C.F. Association of inflammatory factors and aging in Parkinson’s disease. Neurosci. Lett. 2020, 736, 135259.
- Dommershuijsen, L.J.; Ruiter, R.; Erler, N.S.; Rizopoulos, D.; Ikram, M.A.; Ikram, M.K. Peripheral Immune Cell Numbers and C-Reactive Protein in Parkinson’s Disease: Results from a Population-Based Study. J. Parkinsons Dis. 2022, 12, 667–678.
- Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Inflammatory cytokines and NT-proCNP in Parkinson’s disease patients. Cytokine 2012, 60, 762–766.
- Hu, Y.; Yu, S.Y.; Zuo, L.J.; Cao, C.J.; Wang, F.; Chen, Z.J.; Du, Y.; Lian, T.H.; Wang, Y.J.; Chan, P.; et al. Parkinson disease with REM sleep behavior disorder: Features, α-synuclein, and inflammation. Neurology 2015, 84, 888–894.
- Milyukhina, I.V.; Karpenko, M.N.; Klimenko, V.M. Clinical parameters and the level of certain cytokines in blood and cerebrospinal fluid of patients with Parkinson’s disease. Klin. Med. 2015, 93, 51–55.
- Brockmann, K.; Schulte, C.; Schneiderhan-Marra, N.; Apel, A.; Pont-Sunyer, C.; Vilas, D.; Ruiz-Martinez, J.; Langkamp, M.; Corvol, J.C.; Cormier, F.; et al. Inflammatory profile in LRRK2-associated prodromal and clinical PD. Eur. J. Neurol. 2017, 24, 122.
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Miliukhina, I.V.; Bernadotte, A. Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell. Immunol. 2018, 327, 77–82.
- Rocha, N.P.; Assis, F.; Scalzo, P.L.; Vieira, É.L.M.; Barbosa, I.G.; de Souza, M.S.; Christo, P.P.; Reis, H.J.; Teixeira, A.L. Reduced Activated T Lymphocytes (CD4+CD25+) and Plasma Levels of Cytokines in Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 1488–1497.
- Alrafiah, A.; Al-Ofi, E.; Obaid, M.T.; Alsomali, N. Assessment of the Levels of Level of Biomarkers of Bone Matrix Glycoproteins and Inflammatory Cytokines from Saudi Parkinson Patients. Biomed. Res. Int. 2019, 2019, 2690205.
- Lian, T.H.; Guo, P.; Zuo, L.J.; Hu, Y.; Yu, S.Y.; Yu, Q.J.; Jin, Z.; Wang, R.D.; Li, L.X.; Zhang, W. Tremor-Dominant in Parkinson Disease: The Relevance to Iron Metabolism and Inflammation. Front. Neurosci. 2019, 13, 255.
- Chatterjee, K.; Roy, A.; Banerjee, R.; Choudhury, S.; Mondal, B.; Halder, S.; Basu, P.; Shubham, S.; Dey, S.; Kumar, H. Inflammasome and α-synuclein in Parkinson’s disease: A cross-sectional study. J. Neuroimmunol. 2020, 338, 577089.
- Fan, Z.; Pan, Y.T.; Zhang, Z.Y.; Yang, H.; Yu, S.Y.; Zheng, Y.; Ma, J.H.; Wang, X.M. Systemic activation of NLRP3 inflammasome and plasma α-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J. Neuroinflamm. 2020, 17, 11.
- Mahlknecht, P.; Stemberger, S.; Sprenger, F.; Rainer, J.; Hametner, E.; Kirchmair, R.; Grabmer, C.; Scherfler, C.; Wenning, G.K.; Seppi, K.; et al. An antibody microarray analysis of serum cytokines in neurodegenerative Parkinsonian syndromes. Proteome Sci. 2012, 10, 71.
- Schröder, J.B.; Pawlowski, M.; Meyer Zu Hörste, G.; Gross, C.C.; Wiendl, H.; Meuth, S.G.; Ruck, T.; Warnecke, T. Immune Cell Activation in the Cerebrospinal Fluid of Patients With Parkinson’s Disease. Front. Neurol. 2018, 9, 1081.
- Tang, P.; Chong, L.; Li, X.; Liu, Y.; Liu, P.; Hou, C.; Li, R. Correlation between serum RANTES levels and the severity of Parkinson’s disease. Oxid. Med. Cell. Longev. 2014, 2014, 208408.
- Delgado-Alvarado, M.; Gago, B.; Gorostidi, A.; Jiménez-Urbieta, H.; Dacosta-Aguayo, R.; Navalpotro-Gómez, I.; Ruiz-Martínez, J.; Bergareche, A.; Martí-Massó, J.F.; Martínez-Lage, P.; et al. Tau/α-synuclein ratio and inflammatory proteins in Parkinson’s disease: An exploratory study. Mov. Disord. 2017, 32, 1066–1073.
- Kwiatek-Majkusiak, J.; Geremek, M.; Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Serum levels of hepcidin and interleukin 6 in Parkinson’s disease. Acta Neurobiol. Exp. 2020, 80, 297–304.
- Miliukhina, I.V.; Usenko, T.S.; Senkevich, K.A.; Nikolaev, M.A.; Timofeeva, A.A.; Agapova, E.A.; Semenov, A.V.; Lubimova, N.E.; Totolyan, A.A.; Pchelina, S.N. Plasma Cytokines Profile in Patients with Parkinson’s Disease Associated with Mutations in GBA Gene. Bull. Exp. Biol. Med. 2020, 168, 423–426.
- Calvani, R.; Picca, A.; Landi, G.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Arcidiacono, A.; et al. A novel multi-marker discovery approach identifies new serum biomarkers for Parkinson’s disease in older people: An EXosomes in PArkiNson Disease (EXPAND) ancillary study. Geroscience 2020, 42, 1323–1334.
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129.
- Rathnayake, D.; Chang, T.; Udagama, P. Selected serum cytokines and nitric oxide as potential multi-marker biosignature panels for Parkinson disease of varying durations: A case-control study. BMC Neurol. 2019, 19, 56.
- Usenko, T.S.; Nikolaev, M.A.; Miliukhina, I.V.; Bezrukova, A.I.; Senkevich, K.A.; Gomzyakova, N.A.; Beltceva, Y.A.; Zalutskaya, N.M.; Gracheva, E.V.; Timofeeva, A.A.; et al. Plasma cytokine profile in synucleinophaties with dementia. J. Clin. Neurosci. 2020, 78, 323–326.
- Eidson, L.N.; Kannarkat, G.T.; Barnum, C.J.; Chang, J.; Chung, J.; Caspell-Garcia, C.; Taylor, P.; Mollenhauer, B.; Schlossmacher, M.G.; Ereshefsky, L.; et al. Candidate inflammatory biomarkers display unique relationships with alpha-synuclein and correlate with measures of disease severity in subjects with Parkinson’s disease. J. Neuroinflamm. 2017, 14, 164.
- Martin-Ruiz, C.; Williams-Gray, C.H.; Yarnall, A.J.; Boucher, J.J.; Lawson, R.A.; Wijeyekoon, R.S.; Barker, R.A.; Kolenda, C.; Parker, C.; Burn, D.J.; et al. Senescence and Inflammatory Markers for Predicting Clinical Progression in Parkinson’s Disease: The ICICLE-PD Study. J. Parkinsons Dis. 2020, 10, 193–206.
- Csencsits-Smith, K.; Suescun, J.; Li, K.; Luo, S.; Bick, D.L.; Schiess, M. Serum Lymphocyte-Associated Cytokine Concentrations Change More Rapidly over Time in Multiple System Atrophy Compared to Parkinson Disease. Neuroimmunomodulation 2016, 23, 301–308.
- Kouchaki, E.; Kakhaki, R.D.; Tamtaji, O.R.; Dadgostar, E.; Behnam, M.; Nikoueinejad, H.; Akbari, H. Increased serum levels of TNF-α and decreased serum levels of IL-27 in patients with Parkinson disease and their correlation with disease severity. Clin. Neurol. Neurosurg. 2018, 166, 76–79.
- Bagheri, V.; Khorramdelazad, H.; Hassanshahi, G.; Moghadam-Ahmadi, A.; Vakilian, A. CXCL12 and CXCR4 in the Peripheral Blood of Patients with Parkinson’s Disease. Neuroimmunomodulation 2018, 25, 201–205.
- Carvalho, D.Z.; Schönwald, S.V.; Schumacher-Schuh, A.F.; Braga, C.W.; Souza, D.O.; Oses, J.P.; Donis, K.C.; Rieder, C.R. Overnight S100B in Parkinson’s Disease: A glimpse into sleep-related neuroinflammation. Neurosci Lett. 2015, 608, 57–63.
- Dufek, M.; Rektorova, I.; Thon, V.; Lokaj, J.; Rektor, I. Interleukin-6 May Contribute to Mortality in Parkinson’s Disease Patients: A 4-Year Prospective Study. Parkinsons Dis. 2015, 2015, 898192.
- Dumitrescu, L.; Marta, D.; Dănău, A.; Lefter, A.; Tulbă, D.; Cozma, L.; Manole, E.; Gherghiceanu, M.; Ceafalan, L.C.; Popescu, B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021, 15, 689723.
- Green, H.F.; Khosousi, S.; Svenningsson, P. Plasma IL-6 and IL-17A Correlate with Severity of Motor and Non-Motor Symptoms in Parkinson’s Disease. J. Parkinsons Dis. 2019, 9, 705–709.
- Gupta, M.; Paliwal, V.K.; Babu, G.N. Serum fractalkine and 3-nitrotyrosine levels correlate with disease severity in Parkinson’s disease: A pilot study. Metab. Brain Dis. 2022, 37, 209–217.
- Herlofson, K.; Heijnen, C.J.; Lange, J.; Alves, G.; Tysnes, O.B.; Friedman, J.H.; Fagundes, C.P. Inflammation and fatigue in early, untreated Parkinson’s Disease. Acta Neurol. Scand. 2018, 138, 394–399.
- Lerche, S.; Zimmermann, M.; Wurster, I.; Roeben, B.; Fries, F.L.; Deuschle, C.; Waniek, K.; Lachmann, I.; Gasser, T.; Jakobi, M.; et al. CSF and Serum Levels of Inflammatory Markers in PD: Sparse Correlation, Sex Differences and Association With Neurodegenerative Biomarkers. Front. Neurol. 2022, 13, 834580.
- Pereira, J.R.; Santos, L.V.D.; Santos, R.M.S.; Campos, A.L.F.; Pimenta, A.L.; de Oliveira, M.S.; Bacheti, G.G.; Rocha, N.P.; Teixeira, A.L.; Christo, P.P.; et al. IL-6 serum levels are elevated in Parkinson’s disease patients with fatigue compared to patients without fatigue. J. Neurol. Sci. 2016, 370, 153–156.
- Perner, C.; Perner, F.; Gaur, N.; Zimmermann, S.; Witte, O.W.; Heidel, F.H.; Grosskreutz, J.; Prell, T. Plasma VCAM1 levels correlate with disease severity in Parkinson’s disease. J. Neuroinflamm. 2019, 16, 94.
- Rocha, N.P.; Teixeira, A.L.; Scalzo, P.L.; Barbosa, I.G.; de Sousa, M.S.; Morato, I.B.; Vieira, E.L.; Christo, P.P.; Palotás, A.; Reis, H.J. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson’s disease. Mov. Disord. 2014, 29, 527–531.
- Roy, A.; Choudhury, S.; Banerjee, R.; Basu, P.; Kumar, H. Soluble LAG-3 and Toll-interacting protein: Novel upstream neuro-inflammatory markers in Parkinson’s disease. Parkinsonism Relat. Disord. 2021, 91, 121–123.
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory processes in Parkinson’s disease. J. Neural Transm. Suppl. 2006, 70, 373–381.
- Sun, C.; Yu, W.; Zhao, Z.; Song, C.; Liu, Y.; Jia, G.; Wang, X.; Liu, Y. Peripheral humoral immune response is associated with the non-motor symptoms of Parkinson’s disease. Front. Neurosci. 2019, 13, 1057.
- Umemura, A.; Oeda, T.; Yamamoto, K.; Tomita, S.; Kohsaka, M.; Park, K.; Sugiyama, H.; Sawada, H. Baseline Plasma C-Reactive Protein Concentrations and Motor Prognosis in Parkinson Disease. PLoS ONE 2015, 10, e0136722.
- Veselý, B.; Koriťáková, E.; Bohnen, N.I.; Viszlayová, D.; Királová, S.; Valkovič, P.; Kurča, E.; Rektor, I. The contribution of cerebrovascular risk factors, metabolic and inflammatory changes to cognitive decline in Parkinson’s disease: Preliminary observations. J. Neural. Transm. 2019, 126, 1303–1312.
- Yang, F.; Li, B.; Li, L.; Zhang, H. The clinical significance of the imbalance of Th17 and Treg cells and their related cytokines in peripheral blood of Parkinson’s disease patients. Int. J. Clin. Exp. Med. 2016, 9, 17946–17951.
- Yilmaz, R.; Strafella, A.P.; Bernard, A.; Schulte, C.; van den Heuvel, L.; Schneiderhan-Marra, N.; Knorpp, T.; Joos, T.O.; Leypoldt, F.; Geritz, J.; et al. Serum Inflammatory Profile for the Discrimination of Clinical Subtypes in Parkinson’s Disease. Front. Neurol. 2018, 9, 1123.
More
Information
Subjects:
Anesthesiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
607
Revisions:
2 times
(View History)
Update Date:
17 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




