You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | rahul ralegaonkar | -- | 1287 | 2023-05-10 15:50:19 | | | |
| 2 | Catherine Yang | Meta information modification | 1287 | 2023-05-12 03:41:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lanjewar, B.A.; Chippagiri, R.; Dakwale, V.A.; Ralegaonkar, R.V. Alkali-Activated Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/44117 (accessed on 27 December 2025).
Lanjewar BA, Chippagiri R, Dakwale VA, Ralegaonkar RV. Alkali-Activated Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/44117. Accessed December 27, 2025.
Lanjewar, Bhagyashri A., Ravijanya Chippagiri, Vaidehi A. Dakwale, Rahul V. Ralegaonkar. "Alkali-Activated Materials" Encyclopedia, https://encyclopedia.pub/entry/44117 (accessed December 27, 2025).
Lanjewar, B.A., Chippagiri, R., Dakwale, V.A., & Ralegaonkar, R.V. (2023, May 10). Alkali-Activated Materials. In Encyclopedia. https://encyclopedia.pub/entry/44117
Lanjewar, Bhagyashri A., et al. "Alkali-Activated Materials." Encyclopedia. Web. 10 May, 2023.
Copy Citation
Alkali-activated materials (AAM) have been introduced as an eco-friendly alternative to conventional binders with fewer environmental impacts. AAM reduce the need for Ordinary Portland Cement (OPC) by substituting it with supplementary cementitious materials (SCM), and therefore, reducing the amount of subsequent carbon emissions. Alkali activation is a complex chemical process between the precursors (alumino-silicate materials) and their dissolution in the activators. The activator and the precursor are two essential components of AAMs. The first step towards the new generation binder is understanding the precursors, alkali activators, alkali activation solution, and alkali activation mechanism.
alkali-activated materials
alternate binder
sustainable construction material
1. Precursors
Abhishek et al. [1] mentioned that material containing high alumino-silicate and calcium contents can be used as a precursor in the alkali activation process. GGBS, RHA, silica fume, fly ash, metakaolin, bottom ash, corn cob ash, and sugarcane bagasse ash are widely studied precursors [2][3][4] (Table 1). Mostly GGBS and fly ash or a combination of both of them are used as an alumino-silicate-based precursor for alkali-activated concrete [1][5] (Figure 1 and Figure 2). Marvila et al. [6] reported the two types of precursor, one that is rich in alumino-silicates (without calcium oxide), and a second one that is rich in calcium oxide that may or may not contain aluminum oxide (Ca/(Si + Al) ratio higher than one). Nodehi et al. [7] studied the three systems of AAM i.e., high calcium, low calcium (geopolymer), and hybrid (calcium and OPC). Low-calcium-based AAMs generally require oven curing to increase their chemical reactivity, while high-calcium-based AAMs show poor durability properties [7].

Figure 1. Precursors used in AAC.
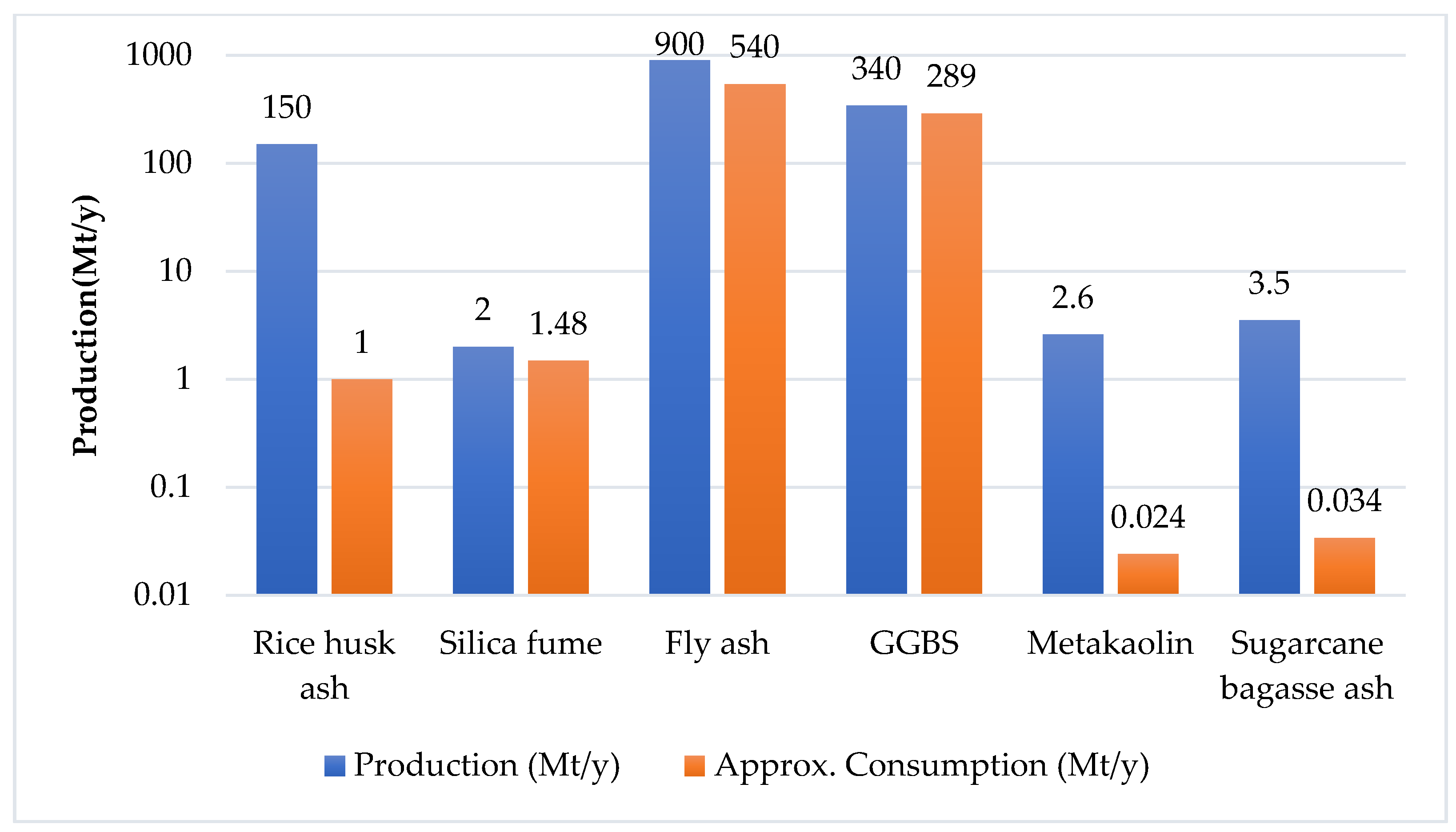
Figure 2. Production and consumption of different precursors [7].
Table 1. Alkali-activated materials.
| Precursors | Molarity of NaOH | Na2SiO3/NaOH | Curing Condition |
Compressive Strength (MPa) | Tensile Strength (Mpa) | Flexural Strength (Mpa) | Remark | Source |
|---|---|---|---|---|---|---|---|---|
| WBG, FA, GBFS, and waste ceramic |
2 M–16 M | 3 | Ambient curing | 54 | - | - | - | [8] |
| Blast Furnace Slag (BFS) + RHA | 12 M | 2 | Ambient curing Thermal Curing |
55 58 |
- - |
6.9 7 |
- | [9] |
| GGBS+FA | 16 M | 2.5 | - | 71 | 4.8 | 7.1 | - | [10] |
| RHA, GGBS, M-Sand, and Copper Slag | 8 M | 1:2.5 | Ambient | 31.1 | 1.95 | - | Inclusion of copper slag of up to 40% is beneficial in chloride aggressive environment | [11] |
| GGBS+FA | 14 M | 1:2.5 | Ambient temperature (27 ± 2 °C) | 54 | 3.8 | 4.9 | Addition of 0.5% of graphene can improve the compressive strength | [12] |
| FA | 8 M–16 M | - | Oven curing | 21.5 | - | 5 | Mechanical properties increased with increase in molarity and decreased with higher Na2SiO3/NaOH ratio | [13] |
| FA | 11.5–13.5 M | - | Ambient | 92.86 | - | - | Strength decreases linearly with the increase in water to alkali activators ratio | [14] |
| GGBS+SF | 13 M | - | - | 32.4 | - | - | M25 grade concrete prepared with 13 Molar NaOH, 40% Na2SiO3 | [15] |
| GGBS+FA (40:60) | 8 M | 1.5 | Ambient temperature (27 ± 2 °C) | 60 | - | - | - | [16] |
| FA | 14 M | 2 | 23 ± 2 °C and Relative humidity 80 ± 5% | 60 | - | - | Replaced conventional aggregate by palm oil clinker aggregate | [17] |
| Metakaolin + Bottom Ash | 8 M | 2 | Ambient curing | 58.95 | 5.96 | 6.94 | - | [18] |
2. Alkali Activators
Depending on the state that is being used, there are two different sorts of activators: liquid activators and solid activators. Alkali-activated materials can be made using one-part alkali activation (dry powder and water) or two-parts alkali activation (dry powder and liquid activator) methods. There are several activators available on the market such as potassium hydroxide, potassium silicate, sodium hydroxide, sodium silicate, sodium carbonate, and sodium sulfate [19][20][21][22][23][24][25] (Figure 3). Sodium metasilicate is the most commonly used solid activator, while sodium hydroxide and sodium silicate are widely used liquid activators (Table 1). Compared to potassium-based activators, sodium-based activators are easily accessible and less expensive. Skin inflammation and other harmful effects have been observed while using potassium hydroxide [7]. The two-part alkali activation is the primary approach used to develop alkali-activated materials, and the majority of research work has been conducted on this only. It can be used for precast work on a large scale, where the chemical handling and temperature for curing can be monitored closely. Researchers are now concentrating on one-part alkali activation, where only the water needs to be added because the transportation part with liquid activators is the major problem. Once the one-part mix method overcomes its limitation, it has the potential for mass production, and it can be distributed as a bagged material.

Figure 3. Common alkali activators used in AAC.
3. Activator Solution
The molarity or concentration of the alkali activator has major influence on the alkali activation process. The relationship between the moles of a solute and the volume of a solution is described by the molarity.
The rate of the dissolution of alumino-silicates depends on the concentration of the alkali solution. Previous studies have shown that a higher molarity increases the dissolution rate. However, a high concentration also reduces the strength, which may be due to the congestion of hydroxide ions (OH−) as a result of the high dissolution rate. Researchers have studied molarity in the range from 4 M to 16 M [26][27], and 8 M–12 M molarity solutions showed the optimal results [10][28][29][30][31]. When they were using two separate activators to achieve the desired results, the ratio of the activators was another key influencing factor. Das et al. [32] investigated the influence of molarity (from 6 M to 16 M) and the alkali activators ratio (1.5, 2.5, and 3.5) on ambient cured concrete. The optimum results were observed with 10 M alkali solution and a 2.5 activators ratio. The further increase in the ratio reduced the flowability and setting time of the mixes by increasing their cohesiveness. The rapid hardening of concrete was observed with an increased ratio of the activators.
4. Alkali Activation Mechanism
4.1. Alkali Activation Mechanism for Low Calcium Precursors
For alkali activation, two essential elements are needed: an activator solution and a solid precursor, which has high silica and alumina contents [33][34][35][36][37][38]. The alkali activation process is the synthesis of the alumino-silicates in strong alkaline media [5]. The alkali activation reaction was divided into four prime steps by Duxson et al. i.e., dissolving, condensation, polycondensation, and gel crystallization [39] (Figure 4). Marvila et al. [6] further explained the Duxson’s alkali activation steps. The dissolution of alumino-silicate materials occurs when the covalent bonds Si-O-Si and Al-O-Al break down in a strong alkaline medium (pH of higher than 14). In other words, a high pH solution breaks the bond between alumino-silicates, resulting in the formation of a colloidal phase [6]. After the dissolution process, the colloidal phase initiates the water elimination process [6][39]. Condensation, a chemical equilibrium process, continues to release water molecules and initiates the gel formation. The gels subsequently undergo a polycondensation process, which increases the number of stable gels that are present, which may or may not lead to crystallization, thereby forming amorphous gels (N-A-S-H) i.e., sodium alumino-silicate hydrate and crystalline or semi-crystalline phases called zeolites [6][40]. Zeolites are made of many interconnected (TO4: T = Si or Al) tetrahedra that have defined pore sizes at the molecular level [6][41][42]. Zeolites can be formed only under specific pressure and temperature conditions (from 25 °C to 300 °C) [6]. The hardening process of the material starts developing the mechanical properties of alkali-activated material.

Figure 4. Alkali activation mechanism for low calcium precursors (Duxson’s model) [43].
4.2. Alkali Activation Mechanism for High Calcium Precursors
The mechanism of alkali activation for high calcium precursors differs from that of the low calcium precursors. They are more reactive at a moderate alkaline pH than the low calcium precursors are, allowing the use of other activators besides sodium or potassium hydroxides and silicates, such as alkali metal carbonate or sulphate solutions [6]. Hydrated calcium silicate gels are predominantly formed as hydration products, which are similar to the hydration products of OPC. The gels have a greater amount of Al present at the tetrahedral locations than Ca, which leads to a higher degree of polymerization. For example, the compound formed in cement hydration is C-S-H, while C-A-S-H gels are formed in the alkali activation of slag [6][44][45]. If excess Al remains present and the material composition contains Mg, then a secondary compound called hydrotalcite is formed. The precursor with low Mg content favors the formation of zeolites over hydrotalcites [6].
References
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh Mechanical and Durability Properties of Alkali-Activated Fly Ash-Slag Concrete: A Review. Innov. Infrastruct. Solut. 2022, 7, 1–14.
- Ruan, Y.; Jamil, T.; Hu, C.; Gautam, B.P.; Yu, J. Microstructure and Mechanical Properties of Sustainable Cementitious Materials with Ultra-High Substitution Level of Calcined Clay and Limestone Powder. Constr. Build. Mater. 2022, 314, 125416.
- Madurwar, M.; Sakhare, V.; Ralegaonkar, R. Suitability and Sustainability of Sugarcane Bagasse Ash Bricks. Proc. Inst. Civ. Eng. Eng. Sustain. 2018, 171, 115–122.
- Saini, G.; Vattipalli, U. Assessing Properties of Alkali Activated GGBS Based Self-Compacting Geopolymer Concrete Using Nano-Silica. Case Stud. Constr. Mater. 2020, 12, e00352.
- Gavali, H.R.; Ralegaonkar, R.V. Design Development of Sustainable Alkali-Activated Bricks. J. Build. Eng. 2020, 30, 101302.
- Ram, S.; Tare, M.S.; Aswath, P.B.; Ralegaonkar, R.V. Potential of Co-Fired Fly Ashes as a Construction Material—A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780128035818.
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2022, 2, 165–196.
- Hao, Y.; Yang, G.; Liang, K. Development of Fly Ash and Slag Based High-Strength Alkali-Activated Foam Concrete. Cem. Concr. Compos. 2022, 128, 104447.
- Jittin, V.; Madhuri, P.; Santhanam, M.; Bahurudeen, A. Influence of Preconditioning and Curing Methods on the Durability Performance of Alkali-Activated Binder Composites. Constr. Build. Mater. 2021, 311, 125346.
- Yaswanth, K.K.; Revathy, J.; Gajalakshmi, P. Strength, Durability and Micro-Structural Assessment of Slag-Agro Blended Based Alkali Activated Engineered Geopolymer Composites. Case Stud. Constr. Mater. 2022, 16, e00920.
- Sajjad, U.; Sheikh, M.N.; Hadi, M.N.S. Incorporation of Graphene in Slag-Fly Ash-Based Alkali-Activated Concrete. Constr. Build. Mater. 2022, 322, 126417.
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of Alkaline Activators on the Mechanical Properties of Fly Ash Based Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2021, 273, 121752.
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A Mix Design Method of Fly Ash Geopolymer Concrete Based on Factors Analysis. Constr. Build. Mater. 2021, 272, 121612.
- Padmakar, M.; Barhmaiah, B.; Priyanka, M.L. Characteristic Compressive Strength of a Geo Polymer Concrete. Mater. Today Proc. 2020, 37, 2219–2222.
- Parihar, H.S.; Verma, M. Analysis on Morality of Polymer Concrete for Enhancing Ambient Temperature. Mater. Today Proc. 2021, 45, 3312–3317.
- Malkawi, A.B.; Habib, M.; Alzubi, Y.; Aladwan, J. Engineering Properties of Lightweight Geopolymer Concrete Using Palm Oil Clinker Aggregate. Int. J. GEOMATE 2020, 18, 132–139.
- Kumar, M.L.; Revathi, V. Microstructural Properties of Alkali-Activated Metakaolin and Bottom Ash Geopolymer. Arab. J. Sci. Eng. 2020, 45, 4235–4246.
- Sheen, Y.N.; Le, D.H. Innovative Use of Sugarcane Bagasse Ash in Green Alkali-Activated Slag Material: Effects of Activator Concentration on the Blended Pastes. Sugar Tech 2022, 24, 1037–1051.
- Reddy, R.S.R.; Anand Sagar, B. Experimental Study on Mechanical and Durability Properties of Recycled Aggregate Based Geo-Polymer Concrete. Mater. Today Proc. 2022, 52, 649–654.
- Nikolić, I.; Karanović, L.; Častvan, I.J.; Radmilović, V.; Mentus, S.; Radmilović, V. Improved Compressive Strength of Alkali Activated Slag upon Heating. Mater. Lett. 2014, 133, 251–254.
- Mathew, G.; Issac, B.M. Effect of Molarity of Sodium Hydroxide on the Aluminosilicate Content in Laterite Aggregate of Laterised Geopolymer Concrete. J. Build. Eng. 2020, 32, 101486.
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. 2014, 62, 32–39.
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical Properties of High Early Strength Class C Fly Ash-Based Alkali Activated Concrete. Transp. Res. Rec. 2020, 2674, 430–443.
- Aydin, S.; Baradan, B. Effect of Activator Type and Content on Properties of Alkali-Activated Slag Mortars. Compos. Part B Eng. 2014, 57, 166–172.
- Parashar, A.K.; Sharma, P.; Sharma, N. An Investigation on Properties of Concrete with the Adding of Waste of Ceramic and Micro Silica. Mater. Today Proc. 2022, 62, 4036–4040.
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of Geopolymer Concrete with GGBFS at Ambient Curing Condition Using Taguchi Method. Constr. Build. Mater. 2017, 140, 424–431.
- Rachmansyah; Hardjasaputra, H.; Cornelia, M. Experimental Study of Effect Additional Water on High Performance Geopolymer Concrete. MATEC Web Conf. 2019, 270, 01004.
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors Affecting the Mechanical Properties of Alkali Activated Ground Granulated Blast Furnace Slag Concrete. Constr. Build. Mater. 2019, 197, 339–355.
- Niş, A. Compressive Strength Variation of Alkali Activated Fly Ash/Slag Concrete with Different NaOH Concentrations and Sodium Silicate to Sodium Hydroxide Ratios. J. Sustain. Constr. Mater. Technol. 2019, 4, 351–360.
- Murugesan, T.; Vidjeapriya, R.; Bahurudeen, A. Development of Sustainable Alkali Activated Binder for Construction Using Sugarcane Bagasse Ash and Marble Waste. Sugar Tech 2020, 22, 885–895.
- Das, S.K.; Shrivastava, S. Influence of Molarity and Alkali Mixture Ratio on Ambient Temperature Cured Waste Cement Concrete Based Geopolymer Mortar. Constr. Build. Mater. 2021, 301, 124380.
- Rekha, Y.; Suriya, S.; Hamedul Irshad, H.M. Comparative Study on Oven Curing of Geo-Polymer Concrete over Conventional Concrete. Mater. Today Proc. 2021, 55, 462–469.
- Shelote, K.M.; Gavali, H.R.; Bras, A.; Ralegaonkar, R.V. Utilization of Co-Fired Blended Ash and Chopped Basalt Fiber in the Development of Sustainable Mortar. Sustainability 2021, 13, 1247.
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329.
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A Review on Alkali-Activated Binders: Materials Composition and Fresh Properties of Concrete. Constr. Build. Mater. 2020, 260, 119788.
- Gómez-Casero, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Steel Slag and Curing Temperature on the Improvement in Technological Properties of Biomass Bottom Ash Based Alkali-Activated Materials. Constr. Build. Mater. 2021, 302, 124205.
- Fu, Q.; Xu, W.; Zhao, X.; Bu, M.X.; Yuan, Q.; Niu, D. The Microstructure and Durability of Fly Ash-Based Geopolymer Concrete: A Review. Ceram. Int. 2021, 47, 29550–29566.
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933.
- Sun, B.; Ye, G.; de Schutter, G. A Review: Reaction Mechanism and Strength of Slag and Fly Ash-Based Alkali-Activated Materials. Constr. Build. Mater. 2022, 326, 126843.
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of Zeolites by In-Situ Conversion of Geopolymers and Their Performance of Heavy Metal Ion Removal in Wastewater:A Review. J. Clean. Prod. 2022, 349, 131441.
- Candamano, S.; Policicchio, A.; Conte, G.; Abarca, R.; Algieri, C.; Chakraborty, S.; Curcio, S.; Calabro, V.; Crea, F.; Agostino, R.G. Preparation of Foamed and Unfoamed Geopolymer/NaX Zeolite/Activated Carbon Composites for CO2 Adsorption. J. Clean. Prod. 2022, 330, 129843.
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The Effect of Alkali and Si/Al Ratio on the Development of Mechanical Properties of Metakaolin-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20.
- Candamano, S.; Crea, F.; Iorfida, A. Mechanical Characterization of Basalt Fabric-Reinforced Alkali-Activated Matrix Composite: A Preliminary Investigation. Appl. Sci. 2020, 10, 2865.
- Angulo-Ramírez, D.E.; Mejía de Gutiérrez, R.; Puertas, F. Alkali-Activated Portland Blast-Furnace Slag Cement: Mechanical Properties and Hydration. Constr. Build. Mater. 2017, 140, 119–128.
- Karim, M.R.; Zain, M.F.M.; Jamil, M.; Lai, F.C. Fabrication of a Non-Cement Binder Using Slag, Palm Oil Fuel Ash and Rice Husk Ash with Sodium Hydroxide. Constr. Build. Mater. 2013, 49, 894–902.
More
Information
Subjects:
Engineering, Civil
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
12 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




