Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by rahul ralegaonkar and Version 2 by Catherine Yang.
Alkali-activated materials (AAM) have been introduced as an eco-friendly alternative to conventional binders with fewer environmental impacts. AAM reduce the need for Ordinary Portland Cement (OPC) by substituting it with supplementary cementitious materials (SCM), and therefore, reducing the amount of subsequent carbon emissions. Alkali activation is a complex chemical process between the precursors (alumino-silicate materials) and their dissolution in the activators. The activator and the precursor are two essential components of AAMs. The first step towards the new generation binder is understanding the precursors, alkali activators, alkali activation solution, and alkali activation mechanism.
- alkali-activated materials
- alternate binder
- sustainable construction material
1. Precursors
Abhishek et al. [1][28] mentioned that material containing high alumino-silicate and calcium contents can be used as a precursor in the alkali activation process. GGBS, RHA, silica fume, fly ash, metakaolin, bottom ash, corn cob ash, and sugarcane bagasse ash are widely studied precursors [2][3][4][43,64,65] (Table 13). Mostly GGBS and fly ash or a combination of both of them are used as an alumino-silicate-based precursor for alkali-activated concrete [1][5][19,28] (Figure 1 and Figure 2). Marvila et al. [6][63] reported the two types of precursor, one that is rich in alumino-silicates (without calcium oxide), and a second one that is rich in calcium oxide that may or may not contain aluminum oxide (Ca/(Si + Al) ratio higher than one). Nodehi et al. [7][32] studied the three systems of AAM i.e., high calcium, low calcium (geopolymer), and hybrid (calcium and OPC). Low-calcium-based AAMs generally require oven curing to increase their chemical reactivity, while high-calcium-based AAMs show poor durability properties [7][32].
Figure 1.
Precursors used in AAC.
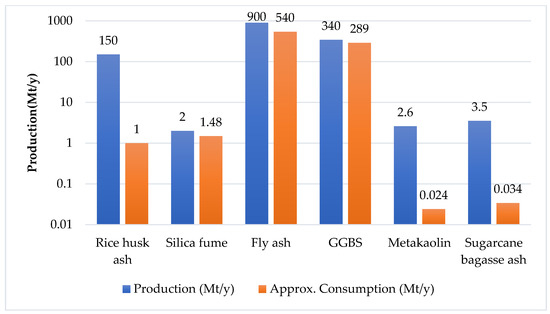
Table 13.
Alkali-activated materials.
| Precursors | Molarity of NaOH | Na2SiO3/NaOH | Curing Condition |
Compressive Strength (MPa) | Tensile Strength (Mpa) | Flexural Strength (Mpa) | Remark | Source |
|---|---|---|---|---|---|---|---|---|
| WBG, FA, GBFS, and waste ceramic |
2 M–16 M | 3 | Ambient curing | 54 | - | - | - | [8][55] |
| Blast Furnace Slag (BFS) + RHA | 12 M | 2 | Ambient curing Thermal Curing |
55 58 |
- - |
6.9 7 |
- | [9][59] |
| GGBS+FA | 16 M | 2.5 | - | 71 | 4.8 | 7.1 | - | [10][66] |
| RHA, GGBS, M-Sand, and Copper Slag | 8 M | 1:2.5 | Ambient | 31.1 | 1.95 | - | Inclusion of copper slag of up to 40% is beneficial in chloride aggressive environment | [11][67] |
| GGBS+FA | 14 M | 1:2.5 | Ambient temperature (27 ± 2 °C) | 54 | 3.8 | 4.9 | Addition of 0.5% of graphene can improve the compressive strength | [12][68] |
| FA | 8 M–16 M | - | Oven curing | 21.5 | - | 5 | Mechanical properties increased with increase in molarity and decreased with higher Na2SiO3/NaOH ratio | [13][69] |
| FA | 11.5–13.5 M | - | Ambient | 92.86 | - | - | Strength decreases linearly with the increase in water to alkali activators ratio | [14][70] |
| GGBS+SF | 13 M | - | - | 32.4 | - | - | M25 grade concrete prepared with 13 Molar NaOH, 40% Na2SiO3 | [15][71] |
| GGBS+FA (40:60) | 8 M | 1.5 | Ambient temperature (27 ± 2 °C) | 60 | - | - | - | [16][72] |
| FA | 14 M | 2 | 23 ± 2 °C and Relative humidity 80 ± 5% | 60 | - | - | Replaced conventional aggregate by palm oil clinker aggregate | [17][73] |
| Metakaolin + Bottom Ash | 8 M | 2 | Ambient curing | 58.95 | 5.96 | 6.94 | - | [18][74] |
2. Alkali Activators
Depending on the state that is being used, there are two different sorts of activators: liquid activators and solid activators. Alkali-activated materials can be made using one-part alkali activation (dry powder and water) or two-parts alkali activation (dry powder and liquid activator) methods. There are several activators available on the market such as potassium hydroxide, potassium silicate, sodium hydroxide, sodium silicate, sodium carbonate, and sodium sulfate [19][20][21][22][23][24][25][75,76,77,78,79,80,81] (Figure 3). Sodium metasilicate is the most commonly used solid activator, while sodium hydroxide and sodium silicate are widely used liquid activators (Table 13). Compared to potassium-based activators, sodium-based activators are easily accessible and less expensive. Skin inflammation and other harmful effects have been observed while using potassium hydroxide [7][32]. The two-part alkali activation is the primary approach used to develop alkali-activated materials, and the majority of research work has been conducted on this only. It can be used for precast work on a large scale, where the chemical handling and temperature for curing can be monitored closely. Researchers are now concentrating on one-part alkali activation, where only the water needs to be added because the transportation part with liquid activators is the major problem. Once the one-part mix method overcomes its limitation, it has the potential for mass production, and it can be distributed as a bagged material.
Figure 3.
Common alkali activators used in AAC.
3. Activator Solution
The molarity or concentration of the alkali activator has major influence on the alkali activation process. The relationship between the moles of a solute and the volume of a solution is described by the molarity.
The rate of the dissolution of alumino-silicates depends on the concentration of the alkali solution. Previous studies have shown that a higher molarity increases the dissolution rate. However, a high concentration also reduces the strength, which may be due to the congestion of hydroxide ions (OH−) as a result of the high dissolution rate. Researchers have studied molarity in the range from 4 M to 16 M [26][27][82,83], and 8 M–12 M molarity solutions showed the optimal results [10][28][29][30][31][39,66,84,85,86]. When they were using two separate activators to achieve the desired results, the ratio of the activators was another key influencing factor. Das et al. [32][87] investigated the influence of molarity (from 6 M to 16 M) and the alkali activators ratio (1.5, 2.5, and 3.5) on ambient cured concrete. The optimum results were observed with 10 M alkali solution and a 2.5 activators ratio. The further increase in the ratio reduced the flowability and setting time of the mixes by increasing their cohesiveness. The rapid hardening of concrete was observed with an increased ratio of the activators.
4. Alkali Activation Mechanism
4.1. Alkali Activation Mechanism for Low Calcium Precursors
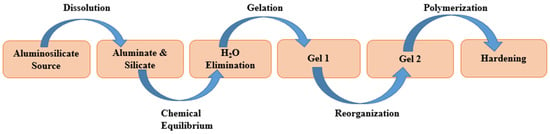
For alkali activation, two essential elements are needed: an activator solution and a solid precursor, which has high silica and alumina contents [33][34][35][36][37][38][20,26,34,88,89,90]. The alkali activation process is the synthesis of the alumino-silicates in strong alkaline media [5][19]. The alkali activation reaction was divided into four prime steps by Duxson et al. i.e., dissolving, condensation, polycondensation, and gel crystallization [39][91] (Figure 4). Marvila et al. [6][63] further explained the Duxson’s alkali activation steps. The dissolution of alumino-silicate materials occurs when the covalent bonds Si-O-Si and Al-O-Al break down in a strong alkaline medium (pH of higher than 14). In other words, a high pH solution breaks the bond between alumino-silicates, resulting in the formation of a colloidal phase [6][63]. After the dissolution process, the colloidal phase initiates the water elimination process [6][39][63,91]. Condensation, a chemical equilibrium process, continues to release water molecules and initiates the gel formation. The gels subsequently undergo a polycondensation process, which increases the number of stable gels that are present, which may or may not lead to crystallization, thereby forming amorphous gels (N-A-S-H) i.e., sodium alumino-silicate hydrate and crystalline or semi-crystalline phases called zeolites [6][40][63,92]. Zeolites are made of many interconnected (TO4: T = Si or Al) tetrahedra that have defined pore sizes at the molecular level [6][41][42][63,93,94]. Zeolites can be formed only under specific pressure and temperature conditions (from 25 °C to 300 °C) [6][63]. The hardening process of the material starts developing the mechanical properties of alkali-activated material.
4.2. Alkali Activation Mechanism for High Calcium Precursors
The mechanism of alkali activation for high calcium precursors differs from that of the low calcium precursors. They are more reactive at a moderate alkaline pH than the low calcium precursors are, allowing the use of other activators besides sodium or potassium hydroxides and silicates, such as alkali metal carbonate or sulphate solutions [6][63]. Hydrated calcium silicate gels are predominantly formed as hydration products, which are similar to the hydration products of OPC. The gels have a greater amount of Al present at the tetrahedral locations than Ca, which leads to a higher degree of polymerization. For example, the compound formed in cement hydration is C-S-H, while C-A-S-H gels are formed in the alkali activation of slag [6][44][45][63,96,97]. If excess Al remains present and the material composition contains Mg, then a secondary compound called hydrotalcite is formed. The precursor with low Mg content favors the formation of zeolites over hydrotalcites [6][63].
