Alkali-activated materials (AAM) have been introduced as an eco-friendly alternative to conventional binders with fewer environmental impacts. AAM reduce the need for Ordinary Portland Cement (OPC) by substituting it with supplementary cementitious materials (SCM), and therefore, reducing the amount of subsequent carbon emissions. Alkali activation is a complex chemical process between the precursors (alumino-silicate materials) and their dissolution in the activators. The activator and the precursor are two essential components of AAMs. The first step towards the new generation binder is understanding the precursors, alkali activators, alkali activation solution, and alkali activation mechanism.
- alkali-activated materials
- alternate binder
- sustainable construction material
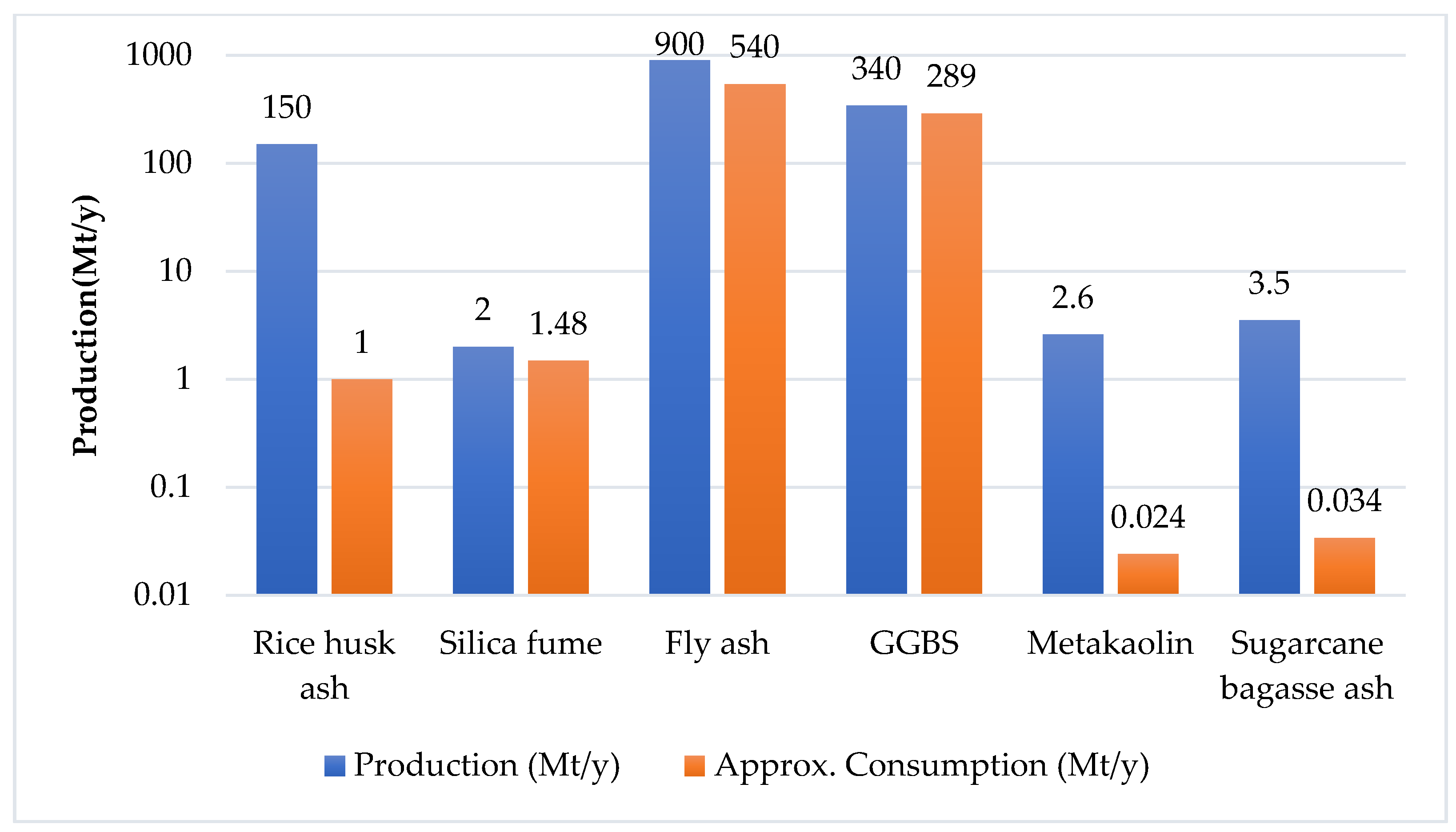
1. Precursors

| Precursors | Molarity of NaOH | Na2SiO3/NaOH | Curing Condition |
Compressive Strength (MPa) | Tensile Strength (Mpa) | Flexural Strength (Mpa) | Remark | Source |
|---|---|---|---|---|---|---|---|---|
| WBG, FA, GBFS, and waste ceramic |
2 M–16 M | 3 | Ambient curing | 54 | - | - | - | [8] |
| Blast Furnace Slag (BFS) + RHA | 12 M | 2 | Ambient curing Thermal Curing |
55 58 |
- - |
6.9 7 |
- | [9] |
| GGBS+FA | 16 M | 2.5 | - | 71 | 4.8 | 7.1 | - | [10] |
| RHA, GGBS, M-Sand, and Copper Slag | 8 M | 1:2.5 | Ambient | 31.1 | 1.95 | - | Inclusion of copper slag of up to 40% is beneficial in chloride aggressive environment | [11] |
| GGBS+FA | 14 M | 1:2.5 | Ambient temperature (27 ± 2 °C) | 54 | 3.8 | 4.9 | Addition of 0.5% of graphene can improve the compressive strength | [12] |
| FA | 8 M–16 M | - | Oven curing | 21.5 | - | 5 | Mechanical properties increased with increase in molarity and decreased with higher Na2SiO3/NaOH ratio | [13] |
| FA | 11.5–13.5 M | - | Ambient | 92.86 | - | - | Strength decreases linearly with the increase in water to alkali activators ratio | [14] |
| GGBS+SF | 13 M | - | - | 32.4 | - | - | M25 grade concrete prepared with 13 Molar NaOH, 40% Na2SiO3 | [15] |
| GGBS+FA (40:60) | 8 M | 1.5 | Ambient temperature (27 ± 2 °C) | 60 | - | - | - | [16] |
| FA | 14 M | 2 | 23 ± 2 °C and Relative humidity 80 ± 5% | 60 | - | - | Replaced conventional aggregate by palm oil clinker aggregate | [17] |
| Metakaolin + Bottom Ash | 8 M | 2 | Ambient curing | 58.95 | 5.96 | 6.94 | - | [18] |
2. Alkali Activators

3. Activator Solution
4. Alkali Activation Mechanism
4.1. Alkali Activation Mechanism for Low Calcium Precursors

4.2. Alkali Activation Mechanism for High Calcium Precursors
This entry is adapted from the peer-reviewed paper 10.3390/en16020969
References
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh Mechanical and Durability Properties of Alkali-Activated Fly Ash-Slag Concrete: A Review. Innov. Infrastruct. Solut. 2022, 7, 1–14.
- Ruan, Y.; Jamil, T.; Hu, C.; Gautam, B.P.; Yu, J. Microstructure and Mechanical Properties of Sustainable Cementitious Materials with Ultra-High Substitution Level of Calcined Clay and Limestone Powder. Constr. Build. Mater. 2022, 314, 125416.
- Madurwar, M.; Sakhare, V.; Ralegaonkar, R. Suitability and Sustainability of Sugarcane Bagasse Ash Bricks. Proc. Inst. Civ. Eng. Eng. Sustain. 2018, 171, 115–122.
- Saini, G.; Vattipalli, U. Assessing Properties of Alkali Activated GGBS Based Self-Compacting Geopolymer Concrete Using Nano-Silica. Case Stud. Constr. Mater. 2020, 12, e00352.
- Gavali, H.R.; Ralegaonkar, R.V. Design Development of Sustainable Alkali-Activated Bricks. J. Build. Eng. 2020, 30, 101302.
- Ram, S.; Tare, M.S.; Aswath, P.B.; Ralegaonkar, R.V. Potential of Co-Fired Fly Ashes as a Construction Material—A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780128035818.
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2022, 2, 165–196.
- Hao, Y.; Yang, G.; Liang, K. Development of Fly Ash and Slag Based High-Strength Alkali-Activated Foam Concrete. Cem. Concr. Compos. 2022, 128, 104447.
- Jittin, V.; Madhuri, P.; Santhanam, M.; Bahurudeen, A. Influence of Preconditioning and Curing Methods on the Durability Performance of Alkali-Activated Binder Composites. Constr. Build. Mater. 2021, 311, 125346.
- Yaswanth, K.K.; Revathy, J.; Gajalakshmi, P. Strength, Durability and Micro-Structural Assessment of Slag-Agro Blended Based Alkali Activated Engineered Geopolymer Composites. Case Stud. Constr. Mater. 2022, 16, e00920.
- Sajjad, U.; Sheikh, M.N.; Hadi, M.N.S. Incorporation of Graphene in Slag-Fly Ash-Based Alkali-Activated Concrete. Constr. Build. Mater. 2022, 322, 126417.
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of Alkaline Activators on the Mechanical Properties of Fly Ash Based Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2021, 273, 121752.
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A Mix Design Method of Fly Ash Geopolymer Concrete Based on Factors Analysis. Constr. Build. Mater. 2021, 272, 121612.
- Padmakar, M.; Barhmaiah, B.; Priyanka, M.L. Characteristic Compressive Strength of a Geo Polymer Concrete. Mater. Today Proc. 2020, 37, 2219–2222.
- Parihar, H.S.; Verma, M. Analysis on Morality of Polymer Concrete for Enhancing Ambient Temperature. Mater. Today Proc. 2021, 45, 3312–3317.
- Malkawi, A.B.; Habib, M.; Alzubi, Y.; Aladwan, J. Engineering Properties of Lightweight Geopolymer Concrete Using Palm Oil Clinker Aggregate. Int. J. GEOMATE 2020, 18, 132–139.
- Kumar, M.L.; Revathi, V. Microstructural Properties of Alkali-Activated Metakaolin and Bottom Ash Geopolymer. Arab. J. Sci. Eng. 2020, 45, 4235–4246.
- Sheen, Y.N.; Le, D.H. Innovative Use of Sugarcane Bagasse Ash in Green Alkali-Activated Slag Material: Effects of Activator Concentration on the Blended Pastes. Sugar Tech 2022, 24, 1037–1051.
- Reddy, R.S.R.; Anand Sagar, B. Experimental Study on Mechanical and Durability Properties of Recycled Aggregate Based Geo-Polymer Concrete. Mater. Today Proc. 2022, 52, 649–654.
- Nikolić, I.; Karanović, L.; Častvan, I.J.; Radmilović, V.; Mentus, S.; Radmilović, V. Improved Compressive Strength of Alkali Activated Slag upon Heating. Mater. Lett. 2014, 133, 251–254.
- Mathew, G.; Issac, B.M. Effect of Molarity of Sodium Hydroxide on the Aluminosilicate Content in Laterite Aggregate of Laterised Geopolymer Concrete. J. Build. Eng. 2020, 32, 101486.
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. 2014, 62, 32–39.
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical Properties of High Early Strength Class C Fly Ash-Based Alkali Activated Concrete. Transp. Res. Rec. 2020, 2674, 430–443.
- Aydin, S.; Baradan, B. Effect of Activator Type and Content on Properties of Alkali-Activated Slag Mortars. Compos. Part B Eng. 2014, 57, 166–172.
- Parashar, A.K.; Sharma, P.; Sharma, N. An Investigation on Properties of Concrete with the Adding of Waste of Ceramic and Micro Silica. Mater. Today Proc. 2022, 62, 4036–4040.
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of Geopolymer Concrete with GGBFS at Ambient Curing Condition Using Taguchi Method. Constr. Build. Mater. 2017, 140, 424–431.
- Rachmansyah; Hardjasaputra, H.; Cornelia, M. Experimental Study of Effect Additional Water on High Performance Geopolymer Concrete. MATEC Web Conf. 2019, 270, 01004.
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors Affecting the Mechanical Properties of Alkali Activated Ground Granulated Blast Furnace Slag Concrete. Constr. Build. Mater. 2019, 197, 339–355.
- Niş, A. Compressive Strength Variation of Alkali Activated Fly Ash/Slag Concrete with Different NaOH Concentrations and Sodium Silicate to Sodium Hydroxide Ratios. J. Sustain. Constr. Mater. Technol. 2019, 4, 351–360.
- Murugesan, T.; Vidjeapriya, R.; Bahurudeen, A. Development of Sustainable Alkali Activated Binder for Construction Using Sugarcane Bagasse Ash and Marble Waste. Sugar Tech 2020, 22, 885–895.
- Das, S.K.; Shrivastava, S. Influence of Molarity and Alkali Mixture Ratio on Ambient Temperature Cured Waste Cement Concrete Based Geopolymer Mortar. Constr. Build. Mater. 2021, 301, 124380.
- Rekha, Y.; Suriya, S.; Hamedul Irshad, H.M. Comparative Study on Oven Curing of Geo-Polymer Concrete over Conventional Concrete. Mater. Today Proc. 2021, 55, 462–469.
- Shelote, K.M.; Gavali, H.R.; Bras, A.; Ralegaonkar, R.V. Utilization of Co-Fired Blended Ash and Chopped Basalt Fiber in the Development of Sustainable Mortar. Sustainability 2021, 13, 1247.
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329.
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A Review on Alkali-Activated Binders: Materials Composition and Fresh Properties of Concrete. Constr. Build. Mater. 2020, 260, 119788.
- Gómez-Casero, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Steel Slag and Curing Temperature on the Improvement in Technological Properties of Biomass Bottom Ash Based Alkali-Activated Materials. Constr. Build. Mater. 2021, 302, 124205.
- Fu, Q.; Xu, W.; Zhao, X.; Bu, M.X.; Yuan, Q.; Niu, D. The Microstructure and Durability of Fly Ash-Based Geopolymer Concrete: A Review. Ceram. Int. 2021, 47, 29550–29566.
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933.
- Sun, B.; Ye, G.; de Schutter, G. A Review: Reaction Mechanism and Strength of Slag and Fly Ash-Based Alkali-Activated Materials. Constr. Build. Mater. 2022, 326, 126843.
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of Zeolites by In-Situ Conversion of Geopolymers and Their Performance of Heavy Metal Ion Removal in Wastewater:A Review. J. Clean. Prod. 2022, 349, 131441.
- Candamano, S.; Policicchio, A.; Conte, G.; Abarca, R.; Algieri, C.; Chakraborty, S.; Curcio, S.; Calabro, V.; Crea, F.; Agostino, R.G. Preparation of Foamed and Unfoamed Geopolymer/NaX Zeolite/Activated Carbon Composites for CO2 Adsorption. J. Clean. Prod. 2022, 330, 129843.
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The Effect of Alkali and Si/Al Ratio on the Development of Mechanical Properties of Metakaolin-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20.
- Candamano, S.; Crea, F.; Iorfida, A. Mechanical Characterization of Basalt Fabric-Reinforced Alkali-Activated Matrix Composite: A Preliminary Investigation. Appl. Sci. 2020, 10, 2865.
- Angulo-Ramírez, D.E.; Mejía de Gutiérrez, R.; Puertas, F. Alkali-Activated Portland Blast-Furnace Slag Cement: Mechanical Properties and Hydration. Constr. Build. Mater. 2017, 140, 119–128.
- Karim, M.R.; Zain, M.F.M.; Jamil, M.; Lai, F.C. Fabrication of a Non-Cement Binder Using Slag, Palm Oil Fuel Ash and Rice Husk Ash with Sodium Hydroxide. Constr. Build. Mater. 2013, 49, 894–902.
