
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kinga Korniejenko | -- | 2392 | 2023-05-04 19:42:58 | | | |
| 2 | Dean Liu | Meta information modification | 2392 | 2023-05-05 03:53:28 | | |
Video Upload Options
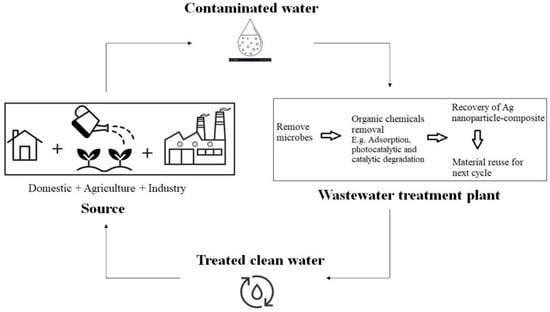
Rapidly increasing industrialisation has human needs, but the consequences have added to the environmental harm. The pollution caused by several industries, including the dye industries, generates a large volume of wastewater containing dyes and hazardous chemicals that drains industrial effluents. The growing demand for readily available water, as well as the problem of polluted organic waste in reservoirs and streams, is a critical challenge for proper and sustainable development. Remediation has resulted in the need for an appropriate alternative to clear up the implications. Nanotechnology is an efficient and effective path to improve wastewater treatment/remediation. The effective surface properties and chemical activity of nanoparticles give them a better chance to remove or degrade the dye material from wastewater treatment. AgNPs (silver nanoparticles) are an efficient nanoparticle for the treatment of dye effluent that have been explored in many studies. The antimicrobial activity of AgNPs against several pathogens is well-recognised in the health and agriculture sectors.
1. AgNPs in Wastewater Management
2. Effects of Nanoparticle Composites in Textile Dye Removal
| No | Nanomaterial Type | Type of Process | Nanoparticle Material | Textile Dyes | Removal Efficiency | References |
|---|---|---|---|---|---|---|
| 1 | Powder | Photocatalytic and microwave-assisted degradation method | ZnO/poly (1-naphthylamine) nanohybrids | Alizarin red | 85% | [12] |
| 2 | Powder | Catalytic degradation method | Pd | Azo dyes | 93 and 91% | [14] |
| 3 | Powder | Catalytic degradation method | Cu | Methyl orange | Less than 80% | [15] |
| 4 | Powder | Photocatalytic degradation method | Fe2O3 | Acid blue | 87% | [16] |
| 5 | Decorated | Enzymatic reaction | Fe3O4 | Acid fuchsin | Up to 80% | [17] |
| 6 | Powder | Photocatalytic degradation method | Ag | Methyl orange and Coomassie brilliant blue |
60%; 70% | [18] |
| 7 | Powder | Biogenic method | Biogenic Pd | Acid blue 1 and red, methyl orange and reactive black 5 |
Less than 95% | [11] |
| 8 | Powder | Photocatalytic degradation method | Ag–ZnO/GO | Methylene blue | 85% | [19] |
| 9 | Powder | Photocatalytic degradation method | ZnO/CuO | Methylene blue | 93% | [20] |
| 10 | Powder | Adsorption | Fe3O4 | Optilan blue | 50 mg/L with 0.6 g/L | [21] |
| 11 | Powder | Desalination | GO-PEG-NB | Ternary dyes | 99% | [22] |
| 12 | Powder | Adsorption–photocatalysis | Ze-nanZnO; nanZnO | Tartrazine | 87 and 81% | [23] |
| 13 | Film | Adsorption | CS/MgO | Reactive blue (RB) 19 | 77.62% | [24] |
| 14 | Powder | Adsorption | CS–ZnO | Malachite green (MG) | 98.5% | [25] |
| 15 | Powder | Photocatalytic degradation method | TiO2 + MC (micro cellulose) | Methylene blue, methyl violet and acid violet | 99% | [26] |
| 16 | Powder | Photo degradation method | CS/ZnO | Methylene blue | CS: 86.7%; MB: 81% | [27] |
| 17 | Powder | Photocatalytic degradation method | ZnO/AC | Methylene blue | 92.2% | [28] |
| 18 | Powder | Adsorption | CHT-GLA/ZnO/Fe3O4 | Brilliant Blue R | 176.6 mg/g at 60 °C | [13] |
| 19 | Ni@FP | Coated on Cellulose filter paper | Dyeing wastewater | Methylene orange | 93.4% | [29] |
| 20 | Dry powdered gel | Photocatalytic degradation | LaFeO3- RGO–NiO |
Congo red | 96.5% | [30] |
| 21 | Powder | Photocatalytic degradation method | Ag–ZnO | Methylene blue, methyl orange and rhodamine B dyes |
98.5% | [31] |
| 22 | Powder | Photocatalytic degradation method | ZnO | Methylene blue | 90% | [32] |
| 23 | Powder | Photocatalytic method | ZnO | Alizarin red S (AZ) and methylene blue (MB) dyes | 99.9 and 96.8% | [33] |
| 24 | Powder | Photocatalytic degradation method | CuO | Methylene blue (MB) | 93% | [34] |
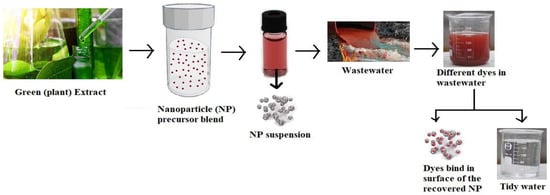
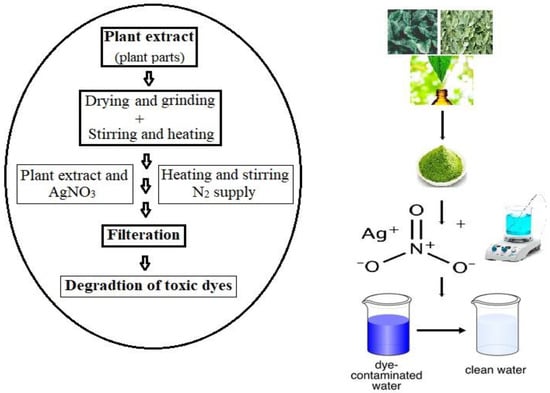
3. Silver Nanoparticles-Composite Activity for Wastewater Treatment
References
- Khan, S.A.; Jain, M.; Pandey, A.; Pant, K.K.; Ziora, Z.M.; Blaskovich, M.A.; Shetti, N.P.; Aminabhavi, T.M. Leveraging the potential of silver nanoparticles-based materials towards sustainable water treatment. J. Environ. Manag. 2022, 319, 115675.
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Aminabhavi, T.M.; Reddy, K.R. Nanostructured silver doped TiO2/CNTs hybrid as an efficient electrochemical sensor for detection of anti-inflammatory drug, cetirizine. Microchem. J. 2019, 150, 104124.
- Tarannum, N.; Divya; Gautam, Y.K. Facile green synthesis and applications of silver nanoparticles: A state-of-the-art review. RSC Adv. 2019, 9, 34926–34948.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Puiatti, G.A.; de Carvalho, J.P.; de Matos, A.T.; Lopes, R.P. Green synthesis of Fe0 nanoparticles using Eucalyptus grandis leaf extract: Characterization and application for dye degradation by a (Photo)Fenton-like process. J. Environ. Manag. 2022, 311, 114828.
- Xiao, C.; Li, H.; Zhao, Y.; Zhang, X.; Wang, X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manag. 2020, 275, 111262.
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246.
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823.
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Technol. 2017, 3, 506–515.
- Łach, M.; Grela, A.; Pławecka, K.; Guigou, M.D.; Mikuła, J.; Komar, N.; Bajda, T.; Korniejenko, K. Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials 2022, 15, 4083.
- Wang, P.-T.; Song, Y.-H.; Fan, H.-C.; Yu, L. Bioreduction of azo dyes was enhanced by in-situ biogenic palladium nanoparticles. Bioresour. Technol. 2018, 266, 176–180.
- Riaz, U.; Ashraf, S.; Budhiraja, V.; Aleem, S.; Kashyap, J. Comparative studies of the photocatalytic and microwave –assisted degradation of alizarin red using ZnO/poly(1-naphthylamine) nanohybrids. J. Mol. Liq. 2016, 216, 259–267.
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Al-Kahtani, A.A.; Alothman, Z.A. Parametric optimization by Box–Behnken design for synthesis of magnetic chitosan-benzil/ZnO/Fe3O4 nanocomposite and textile dye removal. J. Environ. Chem. Eng. 2021, 9, 105166.
- Li, G.; Li, Y.; Wang, Z.; Liu, H. Green synthesis of palladium nanoparticles with carboxymethyl cellulose for degradation of azo-dyes. Mater. Chem. Phys. 2017, 187, 133–140.
- Nagar, N.; Devra, V. Activation of peroxodisulfate and peroxomonosulfate by green synthesized copper nanoparticles for Methyl Orange degradation: A kinetic study. J. Environ. Chem. Eng. 2017, 5, 5793–5800.
- Tharunya, P.; Subha, V.; Kirubanandan, S.; Sandhaya, S.; Renganathan, S. Green synthesis of superparamagnetic iron oxide nanoparticle from Ficus carica fruit extract, characterization studies and its application on dye degradation studies. Asian J. Pharm. Clin. Res. 2017, 10, 125.
- Gao, Z.; Yi, Y.; Zhao, J.; Xia, Y.; Jiang, M.; Cao, F.; Zhou, H.; Wei, P.; Jia, H.; Yong, X.-Y. Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization. Bioresour. Bioprocess. 2018, 5, 27.
- Wang, L.; Lu, F.; Liu, Y.; Wu, Y.; Wu, Z. Photocatalytic degradation of organic dyes and antimicrobial activity of silver nanoparticles fast synthesized by flavonoids fraction of Psidium guajava L. leaves. J. Mol. Liq. 2018, 263, 187–192.
- Thi, V.H.T.; Cao, T.H.; Pham, T.N.; Pham, T.T.; Le, M.C. Synergistic Adsorption and Photocatalytic Activity under Visible Irradiation Using Ag-ZnO/GO Nanoparticles Derived at Low Temperature. J. Chem. 2019, 2019, 2979517.
- Cahino, A.; Loureiro, R.G.; Dantas, J.; Madeira, V.S.; Fernandes, P.C.R. Characterization and evaluation of ZnO/CuO catalyst in the degradation of methylene blue using solar radiation. Ceram. Int. 2019, 45, 13628–13636.
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D.; et al. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73.
- Banerjee, P.; Mukhopadhyay, A.; Das, P. Graphene oxide–nanobentonite composite sieves for enhanced desalination and dye removal. Desalination 2019, 451, 231–240.
- Jawad, A.H.; Mubarak, N.S.A.; Abdulhameed, A.S. Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: Adsorption and mechanism study. Int. J. Biol. Macromol. 2020, 142, 732–741.
- Nga, N.K.; Chau, N.T.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Dev. 2020, 5, 65–72.
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.N. Adsorption of malachite green dye from aqueous solutions using mesoporous chitosan–zinc oxide composite material. Environ. Chem. Ecotoxicol. 2020, 2, 115–125.
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Rangabhashiyam, S.; Khan, M.R.; Alothman, Z.A. Magnetic Chitosan-Glutaraldehyde/Zinc Oxide/Fe3O4 Nanocomposite: Optimization and Adsorptive Mechanism of Remazol Brilliant Blue R Dye Removal. J. Polym. Environ. 2021, 29, 3932–3947.
- Mostafa, M.H.; Elsawy, M.A.; Darwish, M.S.; Hussein, L.I.; Abdaleem, A.H. Microwave-Assisted preparation of Chitosan/ZnO nanocomposite and its application in dye removal. Mater. Chem. Phys. 2020, 248, 122914.
- Mydeen, S.S.; Kumar, R.R.; Sambathkumar, S.; Kottaisamy, M.; Vasantha, V. Facile Synthesis of ZnO/AC Nanocomposites using Prosopis Juliflora for Enhanced Photocatalytic Degradation of Methylene Blue and Antibacterial Activity. Optik 2020, 224, 165426.
- Pandey, A.; Shukla, P.; Srivastava, P.K. Remediation of Dyes in Water using Green Synthesized Nanoparticles (NPs). Int. J. Plant Environ. 2020, 6, 68–84.
- Vijayaraghavan, T.; Althaf, R.; Babu, P.; Parida, K.; Vadivel, S.; Ashok, A.M. Visible light active LaFeO3 nano perovskite-RGO-NiO composite for efficient H2 evolution by photocatalytic water splitting and textile dye degradation. J. Environ. Chem. Eng. 2021, 9, 104675.
- Stanley, R.; Jebasingh, J.A.; Manisha Vidyavathy, S.; Stanley, P.K.; Ponmani, P.; Shekinah, M.; Vasanthi, J. Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik 2021, 231, 166518.
- Soto-Robles, C.; Nava, O.; Cornejo, L.; Lugo-Medina, E.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct. 2021, 1225, 129101.
- Shubha, J.P.; Kavalli, K.; Adil, S.F.; Assal, M.E.; Hatshan, M.R.; Dubasi, N. Facile green synthesis of semiconductive ZnO nanoparticles for photocatalytic degradation of dyes from the textile industry: A kinetic approach. J. King Saud Univ. Sci. 2022, 34, 102047.
- Yasin, A.; Fatima, U.; Shahid, S.; Mansoor, S.; Inam, H.; Javed, M.; Iqbal, S.; Alrbyawi, H.; Somaily, H.H.; Pashameah, R.A.; et al. Fabrication of Copper Oxide Nanoparticles Using Passiflora edulis Extract for the Estimation of Antioxidant Potential and Photocatalytic Methylene Blue Dye Degradation. Agronomy 2022, 12, 2315.
- Alcantara-Cobos, A.; Gutiérrez-Segura, E.; Solache-Ríos, M.; Amaya-Chávez, A.; Solís-Casados, D. Tartrazine removal by ZnO nanoparticles and a zeolite-ZnO nanoparticles composite and the phytotoxicity of ZnO nanoparticles. Microporous Mesoporous Mater. 2020, 302, 110212.
- Rajagopal, S.; Paramasivam, B.; Muniyasamy, K. Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep. Purif. Technol. 2020, 252, 117444.
- Nsom, M.V.; Etape, E.P.; Tendo, J.F.; Namond, B.V.; Chongwain, P.T.; Yufanyi, M.D.; William, N. A Green and Facile Approach for Synthesis of Starch-Pectin Magnetite Nanoparticles and Application by Removal of Methylene Blue from Textile Effluent. J. Nanomater. 2019, 2019, 4576135.
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of zeolite supported nano-metallic catalyst and applications in textile effluent treatment. J. Environ. Chem. Eng. 2020, 8, 104023.
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16.
- Zeng, Q.; Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Huang, L. Facile preparation of recyclable magnetic paper composite materials for efficient photocatalytic degradation of methyl orange. J. Colloid Interface Sci. 2021, 582, 291–300.
- Dubey, S.P.; Dwivedi, A.D.; Kim, I.-C.; Sillanpaa, M.; Kwon, Y.-N.; Lee, C. Synthesis of graphene–carbon sphere hybrid aerogel with silver nanoparticles and its catalytic and adsorption applications. Chem. Eng. J. 2014, 244, 160–167.
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Sci. Rep. 2015, 5, 11873.
- Al-Rawashdeh, N.A.F.; Allabadi, O.; Aljarrah, M.T. Photocatalytic Activity of Graphene Oxide/Zinc Oxide Nanocomposites with Embedded Metal Nanoparticles for the Degradation of Organic Dyes. ACS Omega 2020, 5, 28046–28055.
- Naseem, K.; Rehman, M.Z.U.; Ahmad, A.; Dubal, D.; AlGarni, T.S. Plant Extract Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for Degradation of Toxic Dyes. Coatings 2020, 10, 1235.
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629.
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic Properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735.
- Ghaedi, M.; Heidarpour, S.; Kokhdan, S.N.; Sahraie, R.; Daneshfar, A.; Brazesh, B. Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of Methylene blue: Kinetic and isotherm study of removal process. Powder Technol. 2012, 228, 18–25.
- Das, S.K.; Khan, M.R.; Parandhaman, T.; Laffir, F.; Guha, A.K.; Sekaran, G.; Mandal, A.B. Nano-silica fabricated with silver nanoparticles: Antifouling adsorbent for efficient dye removal, effective water disinfection and biofouling control. Nanoscale 2013, 5, 5549–5560.
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution. Water 2021, 13, 3206.
- Liao, G.; Li, Q.; Zhao, W.; Pang, Q.; Gao, H.; Xu, Z. In-situ construction of novel silver nanoparticle decorated polymeric spheres as highly active and stable catalysts for reduction of methylene blue dye. Appl. Catal. A Gen. 2018, 549, 102–111.
- Karthik, C.; Swathi, N.; Pandi Prabha, S. Green synthesized rGO-AgNP hybrid nanocomposite—An effective antibacterial adsorbent for photocatalytic removal of DB-14 dye from aqueous solution. J. Environ. Chem. Eng. 2020, 8, 103577.
- Heidari, H.; Karbalaee, M. Silver-nanoparticle Supported on Nanocrystalline Cellulose Using Cetyltrimethylammonium Bromide: Synthesis and Catalytic Performance for Decolorization of Dyes. J. Nanostruct. 2021, 11, 48–56.
- Lai, Y.-R.; Lai, J.-T.; Wang, S.S.-S.; Kuo, Y.-C.; Lin, T.-H. Silver nanoparticle-deposited whey protein isolate amyloid fibrils as catalysts for the reduction of methylene blue. Int. J. Biol. Macromol. 2022, 213, 1098–1114.
- Zhang, W.; Wang, X.; Zhang, Y.; van Bochove, B.; Mäkilä, E.; Seppälä, J.; Xu, W.; Willför, S.; Xu, C. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523.
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534.
- Babitha, N.; Christy, S.R.; Palani, G.; Gurumoorthy, M.; Kannan, K.; Chithambaram, V. Enhanced photocatalytic and Antibacterial Activity of Copper oxide Nanoparticles Synthesized by Facile Combustion methods from Mussaendafrondosa Plant Extract. Phys. Chem. Solid State 2020, 23, 443–449.
- Elbakry, S.; Ali, M.E.; Abouelfadl, M.; Badway, N.A.; Salam, K.M. Photocatalytic degradation of organic compounds by TFC membranes functionalized with Ag/rGO nanocomposites. J. Photochem. Photobiol. A Chem. 2022, 430, 113957.




