1. AgNPs in Wastewater Management
The metallic AgNPs have excellent beneficial properties for a wide range of sectors utilized other than wastewater treatment, such as biology, coating, DNA sequencing, food products, drug therapy, cosmetics, biomedicine, and other varieties have been covered [
27,
28]. However, much of the AgNP research focuses on the antimicrobial activity against the several types of microorganisms and is related to water purification, dye removal, and wastewater treatments [
29]. The synthesis of AgNPs can be based on reproducibility and a cost-effective manner, and synthesis methods depend on the differences in reactants and reaction conditions during the process [
30]. Green synthesis of AgNPs from either plants or microorganisms has been surrounded by intra and extracellular approaches. Extracellular methods have commonly been preferred to avoid the difficulty of extracting intracellular AgNPs for down-streaming processing. Biological methods are more environmentally friendly and cost-effective than physical/chemically synthesised methods [
31,
32]. AgNP characterisation has been used to analyse the properties, but the most basic characterisation method for qualitative analysis is to show the visualisation of colour changes [
33]. The degradation of toxic chemicals in aqueous solution using AgNPs has occurred in two ways: first, commonly used AgNPs assist in reducing the contaminants using chemical reducing agents by catalytic reduction. Moreover, AgNPs are used under the induced light degradation method called catalytic degradation [
27].
2. Effects of Nanoparticle Composites in Textile Dye Removal
The sizes range from 1 to 100 nm in different distinctive features that are not found in their bulk configurations. The chemical reactivity of nanoparticles (NPs) in all fields is attributed to the significance of high available surface area. The new advancement in this regard makes use of combined membranes with biogenic nanoparticles to degrade toxic chlorinated mixtures. Functional classes of nanoparticles such as carbonaceous, zeolite, dendrimers, and metal-containing nanoparticles are used in the process of waste purification [
34]. Dendrimer ultrafilters apply more strong working pressures in high-molecular-mass solutes in the range between 1000 and 3000 Da than micro- and nanofillers. Metal-containing nanoparticles play the antibacterial activity against gram-positive bacteria and have a negative and efficient method to kill the number of bacteria and biocides [
35]. In addition, heavy metals are easily removed from arsenic and halogens. Zeolite removes heavy metals from water as an ion-exchange medium [
36]. Carbonaceous substances can act as sorbents in an aqueous environment in organic solutes. Experimental studies have discussed better-performing enzyme reactions that perform better in biologically synthesised functionalized nanoparticles with a membrane than enhanced nanoparticle stability with single-phase reaction [
34].
Table 1 explains the tabulated nanoparticles for textile dye removal. The fabricated nanoparticles were prepared by conventional methods as well as from several textile dyes with ranges from 65 to 99% through catalytic and photocatalytic degradation processes. Additionally, novel degradation processes, such as enzymatic and biogenic processes, showed 80–95% textile dye degradation [
37]. The novel combination degradation method, involving photocatalytic and microwave-assisted methods, showed better removal efficiency (85%) for textile dye [
38]. Parametrically optimised synthesis and adsorptive performance for the magnetic nanocomposite of chitosan-benzil/ZnO/Fe
3O
4 showed the best removal recorded in 98.8% of Remazol Brilliant Blue R dye (RBBR). The adsorptive mechanism in this nanocomposite explained the multi-interactions that are electrostatic attractions, hydrogen and H bonding, and interactions of n–π and π–π. This nanocomposite is suggested to be a promising composite in biosorption for the removal of anionic dyes from an aqueous environment [
39].
Table 1. Nanoparticles for textile dye removal.
| No |
Nanomaterial Type |
Type of Process |
Nanoparticle Material |
Textile Dyes |
Removal Efficiency |
References |
| 1 |
Powder |
Photocatalytic and microwave-assisted degradation method |
ZnO/poly (1-naphthylamine) nanohybrids |
Alizarin red |
85% |
[38] |
| 2 |
Powder |
Catalytic degradation method |
Pd |
Azo dyes |
93 and 91% |
[40] |
| 3 |
Powder |
Catalytic degradation method |
Cu |
Methyl orange |
Less than 80% |
[41] |
| 4 |
Powder |
Photocatalytic degradation method |
Fe2O3 |
Acid blue |
87% |
[42] |
| 5 |
Decorated |
Enzymatic reaction |
Fe3O4 |
Acid fuchsin |
Up to 80% |
[43] |
| 6 |
Powder |
Photocatalytic degradation method |
Ag |
Methyl orange and
Coomassie
brilliant blue |
60%; 70% |
[44] |
| 7 |
Powder |
Biogenic method |
Biogenic Pd |
Acid blue 1 and red,
methyl orange and reactive black 5 |
Less than 95% |
[37] |
| 8 |
Powder |
Photocatalytic degradation method |
Ag–ZnO/GO |
Methylene blue |
85% |
[45] |
| 9 |
Powder |
Photocatalytic degradation method |
ZnO/CuO |
Methylene blue |
93% |
[46] |
| 10 |
Powder |
Adsorption |
Fe3O4 |
Optilan blue |
50 mg/L with 0.6 g/L |
[47] |
| 11 |
Powder |
Desalination |
GO-PEG-NB |
Ternary dyes |
99% |
[48] |
| 12 |
Powder |
Adsorption–photocatalysis |
Ze-nanZnO; nanZnO |
Tartrazine |
87 and 81% |
[49] |
| 13 |
Film |
Adsorption |
CS/MgO |
Reactive blue (RB) 19 |
77.62% |
[50] |
| 14 |
Powder |
Adsorption |
CS–ZnO |
Malachite green (MG) |
98.5% |
[51] |
| 15 |
Powder |
Photocatalytic degradation method |
TiO2 + MC (micro cellulose) |
Methylene blue, methyl violet and acid violet |
99% |
[52] |
| 16 |
Powder |
Photo degradation method |
CS/ZnO |
Methylene blue |
CS: 86.7%; MB: 81% |
[53] |
| 17 |
Powder |
Photocatalytic degradation method |
ZnO/AC |
Methylene blue |
92.2% |
[54] |
| 18 |
Powder |
Adsorption |
CHT-GLA/ZnO/Fe3O4 |
Brilliant Blue R |
176.6 mg/g at 60 °C |
[39] |
| 19 |
Ni@FP |
Coated on Cellulose filter paper |
Dyeing wastewater |
Methylene orange |
93.4% |
[55] |
| 20 |
Dry powdered gel |
Photocatalytic degradation |
LaFeO3-
RGO–NiO |
Congo red |
96.5% |
[56] |
| 21 |
Powder |
Photocatalytic degradation method |
Ag–ZnO |
Methylene blue, methyl orange and
rhodamine B dyes |
98.5% |
[57] |
| 22 |
Powder |
Photocatalytic degradation method |
ZnO |
Methylene blue |
90% |
[58] |
| 23 |
Powder |
Photocatalytic method |
ZnO |
Alizarin red S (AZ) and methylene blue (MB) dyes |
99.9 and 96.8% |
[59] |
| 24 |
Powder |
Photocatalytic degradation method |
CuO |
Methylene blue (MB) |
93% |
[60] |
A Schiff base cross-linked hybrid inorganic–organic synthesised nanocomposite (CS-GLA/TNC) showed an effective bio-absorbent and improved the removability of reactive azo dyes (RR120 dye) from an aqueous environment. It achieved the highest adsorption capacity recorded at 103.1 mg/g and involved the mechanism of many interactions (electrostatic attraction, n–π stacking, and H bonding) [
49]. The magnetic Schiff base nanocomposite of CHT-GLA/ZnO/Fe
3O
4 (chitosan-glutaraldehyde/zincoxide/Fe
3O
4) was fabricated to remove the dye in Remazol Brilliant Blue R through an effective mechanism of adsorption. The Box–Behnken design-based optimisation method was employed for the fabrication of the magnetic adsorbent against dye degradation. This study showed that the highest RBBR-removal efficiency (75.8%) was achieved
using the multi-interaction mechanism [
52,
61]. Alcantara-Cobos et al. [
62] studied the coupled process of adsorption and photocatalytic degradation (adsorption–photocatalysis). The tartrazine removal study explained the preparation of ZnO nanoparticles and zeolite-ZnO composites for a coupled (adsorption–photocatalysis) process. The ZnO nanoparticles (nanoZnO) showed better efficiency compared to the composite in the processes of adsorption and degradation inclusive of UV light. Furthermore, nanoZnO was difficult to
remove from the aqueous solution [
49].
During the degradation of the photocatalytic process in methylene blue, methyl violet (cationic) and acid violet (anionic) dyes were synthesised by synthesised TiO
2 doped on microcellulose nanocomposite (TiO
2 + MC). This study showed that the combination of photocatalytic degradation of TiO
2 + MC + H
2O
2 with the hydrogen peroxide-assisted process removed 200 mg/L (99%) of methylene blue (MB) in 150 min, and 6–7 h were required to complete the removal of the methyl violet and acid violet dyes. The mechanism of dye degradation is combined with adsorption and direct photocatalytic oxidation (by hydroxyl radicals (OH)) by nanocomposite (TiO
2 + MC). The integrated process of AOPs (advanced oxidised process) followed by adsorption, biological treatment, and sand filtration is widely used for complete industrial wastewater [
62]. The nanocomposite of single molecular pectin-starch magnetite hybrid nanoparticles showed higher efficiency of removing methylene blue dye from an aqueous solution. This adsorption depends on temperature and pH, and the hybrid decomposes magnetite temperatures between 250 and 550 °C. The developed nanocomposite showed higher adsorption efficiency and additional benefits such as lower polymer concentration, ease of synthesis, cost-effectiveness, environmental friendliness, and the absence of secondary pollutants [
63].
Physical, chemical, and biological methods are receiving less attention due to their high costs, low efficiency, and low biodegradability. Rashid et al. [
64] explained that the advanced oxidation process (AOP) is another method of removing/degrading dyes from industrial effluents [
64].
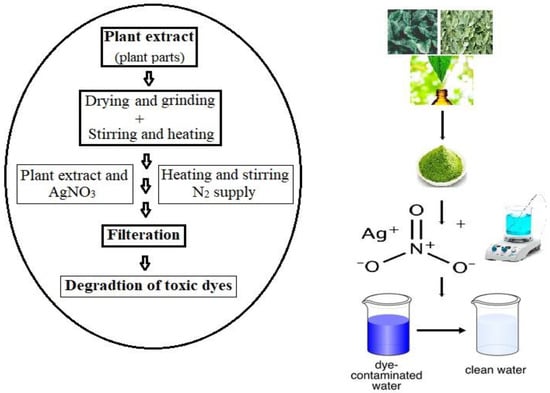
Figure 1 shows the general hypothesis behind the removal of nanoparticles and dyes. The AOP discussed in determining the dye degradation/removal of organic contaminants of the dyes is oxidized by highly reactive species, which are OH (hydroxy radicals), H
2O
2 (hydrogen peroxide), SO
4 (sulfate radical), and O
3 (ozone). The above-mentioned process (AOP) provides satisfactory or potential degradation of dyes from industrial effluents and other contaminants, unlike another conventional process [
3]. The fabrication of a Ni nanoparticle coated with filter paper (Ni@FP material) showed strong magnetic ability and strong antibacterial activity, explaining that an optimum photocatalytic degradation reached 93.4%. This study showed a low-cost material composite (Ni@FP) [
65].
Figure 1. Hypothesis behind the synthesis of nanoparticle and dye remediation (adapted with permission from [
55]).
3. Silver Nanoparticles-Composite Activity for Wastewater Treatment
The role of the noble material silver has been studied and used in different fields of applications focused mainly on medicine and water treatment. Now, silver has rebuilt its character and performance in various forms as a nanoparticle. The biological/green synthesis of AgNPs reforms and maintains a safe environment from harmful works created by the enormous utilisation of chemicals (organic/inorganic) and
addition of metal salt. Furthermore, the silver NP supplies are free from the use of stabilizing agents in the
manufacturing system for chemical and physical processes [
27,
36]. Several research studies have discussed that the fabrication of silver nanoparticles (NPs) from various natural/biological fields and their application in the effluent/wastewater removed dyes.
The fabricated hybrid aerogel graphene–carbon sphere decorated with AgNPs (G/AgCS) used the reduction of anionicdye (CR/congo red) and cationic dye (MB/methylene blue) in the presence of NaBH
4. Furthermore, hydrogels supported by the prepared reduced graphene oxide in polyethyleneimine (PEI) have been utilised to examine the degradation (photocatalytic) of methylene blue and rhodamine B solutions [
66,
67,
68]. Silver NPs are capable of being used for the fast destruction/degradation of organic pollutants reduced into toxic/harmful materials [
27]. Induced biogenic AgNPs extracted from Citrus paradisi degrade and speed up the reduction rate of toxic chemicals in the textile industry wastewater [
69]. The fabricated silver nanocomposites (Ag@MGO-TA/Fe
3+) showed excellent performance of catalytic reduction and antimicrobial activity [
70]. The piezoelectric thin film (FTO/BaTiO
3/AgNPs) produced by the tape-casting method with the deposition of barium titanate/AgNPs degraded the pollutants of methylene blue and ciprofloxacin (pharmaceutical) in wastewater using piezo-photocatalytic degradation. The AgNPs and nanocomposites described above show great potential for several environmental applications with functional implications.
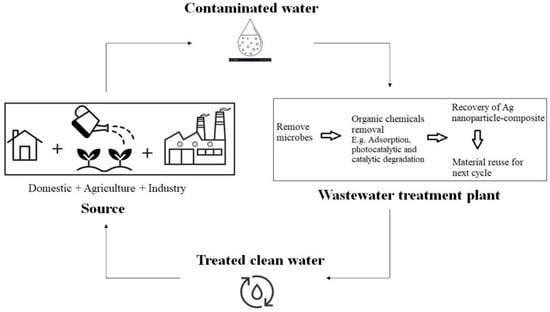
Figure 2 shows the flowchart of silver nanoparticle–composite-treated wastewater for various industries. Metal nanoparticle-based nanocomposites with graphene oxide (GO) have acquired a wide range of potential applications in a number of material science fields. An efficient photocatalyst supported on nanocomposite (GO/ZnO) with metal nanoparticles was synthesised by the one-pot method. The synthesized GO–ZnO–Ag nanocomposite achieved 100% MB dye removal at 40 min of sunlight irradiation. Thus, the silver-based nanocomposite shows potential to be an effective photocatalyst for organic dyes in industrial effluents/wastewater [
68].
Figure 2. Flowchart for the silver nanoparticle compound for wastewater treatment (adapted from [
27]).
The dye removal mechanism using AgNPs includes the adsorption onto AgNPs combined with loaded activated carbon or degradation through catalytic/photocatalytic methods or in combination with both. The addition of silica spheres is used to support the nanoparticles, which avoid the poor catalytic efficiency for the flocculation of nanodimensional materials during the processes of catalytic degradation processes using AgNPs [
92]. Activated carbon loaded with AgNPs was suggested to have high adsorption activity (71.4 mg of MB/g of adsorbent) against methylene blue [
93]. The fabrication of AgNPs with nanosilica powder showed 99% removal of dyes such as Eosin yellow, Bromophenol blue 2, CR, and BR upon adsorption. The desorption studies applying acetone showed at 86% desorption of dye, suggesting the novel adsorbent reusability [
94]. The nanocomposite of Ag/PSNM (silver/poly (styrene-N-isopropylacrylamide-methacrylicacid)) spheres with catalytic degradation of organic dyes showed high potential application for wastewater treatment [
80,
87]. Choudhary et al. [
80] developed and studied biological/green extracts using a silver nanocomposite with naturally occurring montmorillonite (MMT) clay (MMT/Ag nanocomposite). The author investigated the adsorption efficiency and removal of MB dye by applying a batch system. This study revealed that the adsorption of two nanocomposites which were raw MMT and MMT/Ag had the capacity to remove MB (methylene blue) [
80].
The green synthesised hybrid nanocomposite (Brassica nigra) of rGO-AgNP showed antimicrobial activity and efficient photocatalytic activity in direct blue 14 (DB-14) dye. It exhibited a high photocatalysis performance in dye removal with sunlight compared to ultraviolet (UV) and could be reused for five times without a significant loss of photocatalysis performance [
95]. The ultrasonic synthesised Ag/CTAB/NCC (nanohybrid) without acid hydrolysis had a stronger catalytic property than other catalysts and showed better removal of methyl orange (k = 14.2 × 10
−3 s
−1, t = 150 s) and 4-nitophenol (k = 5.4 × 10
−3 s
−1, t = 180 s) [
88]. The one-dimensional AgNP/WPI-AF (amyloid-based hybrid) materials were fabricated using photochemical/chemical routes. The selective support of the AgNP (silver nanoparticle) amyloid fibril (AF) was derived from WPI (whey protein isolate) for the catalytic reduction/removal of the MB (methylene blue) dye. The material of the nanoparticle-amyloid fibril composite is a better example of the process of catalysis, and it showed better reusability [
85]. The fabricated nanocomposite of Ag@MGO-TA/Fe
3+ showed catalytic reduction performance against organic pollutants and antimicrobial performances, especially disinfection action against bacteria (
E. coli) [
70].
The preparation of CNF/PEI/AgNP composites was developed from the cellulose nanofiber (CNF) from cross-linked bleached birch kraft pulp with polyethene imine (PEI) and decorated with silver nanoparticles (AgNPs). It exhibited shape memory properties and good mechanical stability under wet conditions, and its decolorization activity was high as 5 × 10
4 Lm
−2 h
−1. This study demonstrated the recyclability and stability of the 3D nanocellulose-based aerogel membrane after a continuous catalytic discoloration process was performed ten times [
79,
96,
97]. In organic compound degradation, semiconductor nanomaterials are widely used as the photocatalyst. During the photodegradation, the nanoparticles were separated from the treated solution. Therefore, to avoid this problem, the author developed a novel cross-linked membrane and achieved fast degradation of 98% for the Ag/rGO nanocomposite and 92% for Ag/rGO/CA/TFC membranes [
78].
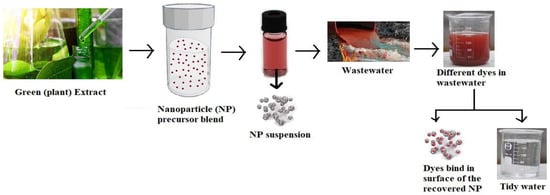
Figure 3 shows a schematic representation of AgNPs (silver nanoparticles) from a plant extract and their use as dye degradation.
Figure 3. Diagrammatic representation of AgNPs (SNPs) from plant extract and their use as a dye degradation (adapted from [
69]).
This entry is adapted from the peer-reviewed paper 10.3390/molecules28083520