
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nunzia Gallo | -- | 4354 | 2023-05-04 10:29:47 | | | |
| 2 | Lindsay Dong | Meta information modification | 4354 | 2023-05-06 07:31:14 | | |
Video Upload Options
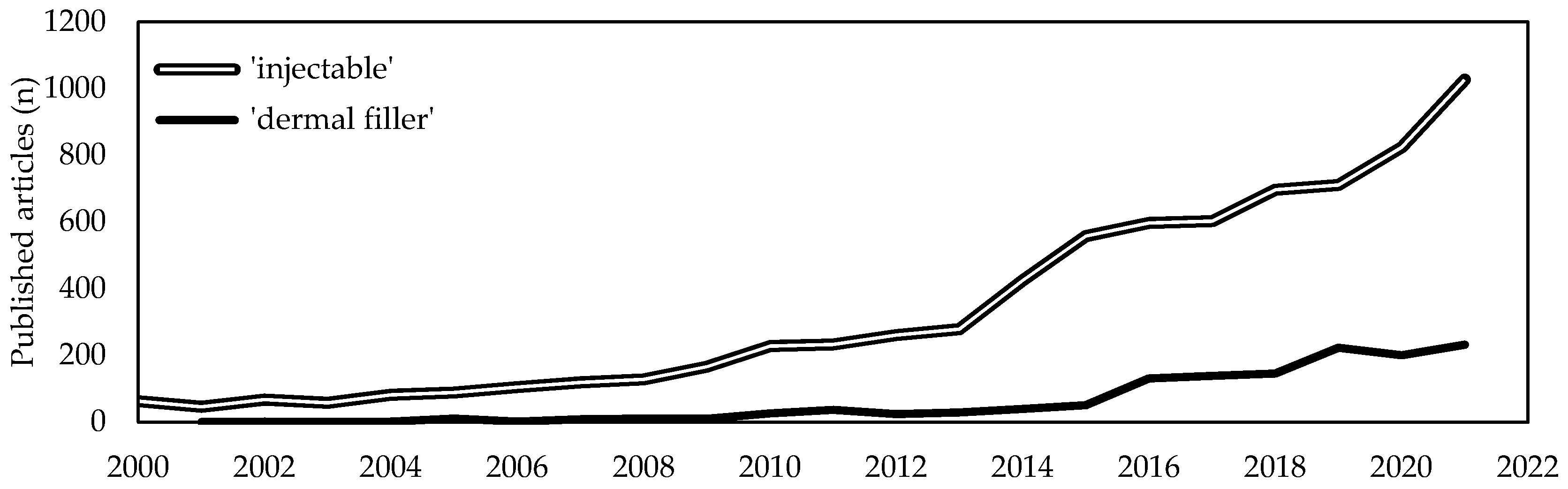
Soft tissues diseases significantly affect patients quality of life and usually require targeted, costly and sometimes constant interventions. With the average lifetime increase, a proportional increase of age-related soft tissues diseases has been witnessed. Due to this, the last two decades have seen a tremendous demand for minimally invasive one-step resolutive procedures. Intensive scientific and industrial research has led to the recognition of injectable formulations as a new advantageous approach in the management of complex diseases that are challenging to treat with conventional strategies. Among them, collagen-based products are revealed to be one of the most promising among bioactive biomaterials-based formulations. Collagen is the most abundant structural protein of vertebrate connective tissues and, because of its structural and non-structural role, is one of the most widely used multifunctional biomaterials in the health-related sectors, including medical care and cosmetics. Indeed, collagen-based formulations are historically considered as the “gold standard” and from 1981 have been paving the way for the development of a new generation of fillers. A huge number of collagen-based injectable products have been approved worldwide for clinical use and have routinely been introduced in many clinical settings for both aesthetic and regenerative surgery.
1. Introduction
2. Collagen as Biomaterial
3. Collagen-Based Injectable Formulations
More than 60 kinds of collagen-based fillers are available on the market, according to the end-use and they have routinely been introduced in many clinical settings (Table 1). The most common collagen extraction sources for the manufacture of collagen based injectable formulations are bovine, swine, porcine, equine and human derived, whose advantages and disadvantages are described in depth elsewhere [19][20][25]. Bovine collagen is one of the most commonly used fillers for effectively reducing wrinkles and other facial imperfections. More famous branded bovine-based collagen fillers are Zyderm®, Zyplast®, Contigen® (Allergan Inc., Dublin, Ireland), Artefill® (Suneva Medical, San Diego, CA, USA), and Artecoll® (Canderm Pharma Inc., Saint-Laurent, QB, Canada). Others include CHondroGrid® (Bioteck Spa, Arcugnano, Italy), Integra Flowable Wound Matrix® (Integra LifeScience Corp., Princeton, NJ, USA), Resoplast® (Rofil Medical International, Breda, The Netherlands), Atelocell® (KOKEN Co., Ltd., Bunkyo-ku, Tokyo, Japan). However, bovine collagen is known to be exposed to zoonosis (e.g., the foot and mouth disease and the group of the bovine spongiform encephalopathies, among which the most dangerous for humans is the transmissible spongiform encephalopathy) and to trigger allergies (about 2–4% of population) [49][50][51]. In addition to the strict regulation to which all implantable products are subjected, two consecutive negative patient skin tests at 6 and 2 weeks are required before use [51][52]. This sensitivity has been considered generally acceptable for implants for human use and actually bovine collagen is principally used for the treatment of the integumental [6][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74] (NCT01060943) and musculoskeletal apparatus [75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90] and to a minor extent for the gastrointestinal [91][92][93][94][95][96][97][98], urinary [99][100][101][102][103][104] and cardiovascular [105][106][107] systems. Recently, bovine collagen in fibrillar form has been employed as an organ protection system during thermal ablation of hepatic malignancies [108].
| Source | Manufacturer | Product | Additives | Applications | Ref. |
|---|---|---|---|---|---|
| Equine | Euroresearch S.r.l. (Milan, Italy) www.euroresearch.it, accessed on 14 February 2023 |
Nithya | – | Integumental | [109] |
| Linerase | – | Integumental | [110][111][112][113][114] | ||
| Nearmedic Italy S.r.l. (Como, Italy) www.salvecoll.com, accessed on 14 February 2023 |
Salvecoll-E | – | Integumental | [115] | |
| Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 |
Biocollagen gel | Type III collagen, bone spongy powder | Musculoskeletal | – | |
| Biocollagen crunch | Type III collagen, bone powder, bone spongy chips |
Musculoskeletal | – | ||
| ActivaBone CLX gel | Bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Injectable Paste |
Demineralized bone matrix, bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone modulable paste |
Demineralized bone matrix, bone powder, bone cortical and spongy granules, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Crunch | Demineralized bone matrix, bone powder, cortical and spongy chips, exur, Vitamin C | Musculoskeletal | – | ||
| Bovine | Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 |
CHondroGrid | – | Musculoskeletal | [90] |
| Integra LifeScience Corp. (Princeton, NJ, USA) www.integralife.com, accessed on 14 February 2023 |
Integra Flowable Wound Matrix | Glycosaminoglycans | Integumental | [66] | |
| Helitene | – | Soft tissues | [108] | ||
| Rofil Medical International (Breda, The Netherlands) | Resoplast | Lidocaine hydrochloride | Integumental | – | |
| Suneva Medical (San Diego, CA, USA) www.sunevamedical.com, accessed on 14 February 2023 |
ArteFill | Polymethylmethacrylate, lidocaine | Integumental | [53][55][56][57][58][59][60][61][62][63] | |
| Datascope Corp., (Montvale, NJ, USA) | VasoSeal | – | Cardiovascular | [107] | |
| BioMimetic Therapeutics, LLC (Franklin, TN, USA) www.biomimetics.com, accessed on 14 February 2023 |
Augment | β-tricalcium phosphate, recombinant human platelet-derived growth factor-BB | Musculoskeletal | [75][77][78][79][80][81][82][83][84][85][86][87][88][89] | |
| KOKEN Co., Ltd. (Bunkyo-ku, Tokyo, Japan) www.kokenmpc.co.jp, accessed on 14 February 2023 |
Atelocell | Type III collagen | Integumental, gastrointestinal |
[64][65][91][92], NCT01060943 | |
| B. Braun (Crissier, Switzerland) www.bbraun.com, accessed on 14 February 2023 |
Gelofusine | – | Cardiovascular | [105][106] | |
| Allergan, Inc. (Dublin, Ireland) www.abbvie.it, accessed on 14 February 2023 |
Zyplast | Glutaraldehyde | Integumental | [6][54][61][67][68][69][70][73][74][76][94][95][97][116] | |
| Zyderm | – | Integumental | [6][61][67][68][71][72][96][98][116] | ||
| Contigen | glutaraldehyde | Gastrointestinal and genitourinary | [93][100][101][102][103][104] | ||
| Swine | GUNA (Milan, Italy) www.guna.com, accessed on 14 February 2023 |
Dental Skin BioRegulation |
Vitamin C, magnesium gluconate, pyridozine chlorhydrate, riboflavin, thiamine chlorhydrate | Skin | [117] |
| Dental ATM BioRegulation |
Hypericum | Musculoskeletal | [118] | ||
| MD-HIP | Calcium phosphate | Musculoskeletal | [119] | ||
| MD-ISCHIAL | Rhododendron | Musculoskeletal | [120] | ||
| MD-KNEE | Arnica | Musculoskeletal | [121][122][123] | ||
| MD-LUMBAR | Hamemelis | Musculoskeletal | [120][124][125] | ||
| MD-NECK | Silicio | Musculoskeletal | – | ||
| MD-SHOULDERS | Iris | Musculoskeletal | [126][127] | ||
| MD-SMALL JOINTS | Viola | Musculoskeletal | – | ||
| MD-THORACIC | Cimifuga | Musculoskeletal | – | ||
| MD-MATRIX | Citric acid, nicotinamide | Soft tissues | [125][128][129] | ||
| MD-MUSCLE | Hypericum | Musculoskeletal | [118][120][121][124][125][127][128][129][130] | ||
| MD-POLY | Drosera | Musculoskeletal | – | ||
| MD-NEURAL | Citrullus | Musculoskeletal | [120][124][129] | ||
| MD-TISSUE | Ascorbic acid, magnesium gluconate, pyridoxine chlorhydrate, riboflavin, thiamine chlorhydrate | Soft tissues | – | ||
| Joint Biomaterials S.r.l. (Mestre, Italy) www.joint-biomateriali.it, accessed on 14 February 2023 |
CartiRegen | Fibrin glue | Musculoskeletal | – | |
| Ubiosis (Gyeonggi-do, Republic of Korea) www.ubiosis.com, accessed on 14 February 2023 |
COLTRIX CartiRegen | – | Musculoskeletal | – | |
| COLTRIX TendoRegen | – | Musculoskeletal | – | ||
| Sewon Cellontech Co., Ltd. (Seoul, Republic of Korea) www.swcell.com, accessed on 14 February 2023 |
CartiFill | Glucose, CaCl, amino acids, vitamin B, fibrin glue |
Musculoskeletal | [131][132], NCT02539030, NCT02519881 | |
| CartiZol | Glucose, CaCl, amino acids, vitamin B | Musculoskeletal | [133], NCT02539095 | ||
| RegenSeal | – | Musculoskeletal | [134] | ||
| TheraFill | – | Integumental | [64][65] | ||
| Sunmax Biotechnology Co., Ltd. (Tainan, Taiwan) www.sunmaxbiotech.com, accessed on 14 February 2023 |
Facial Gain | Lidocaine | Integumental | NCT03844529 | |
| Collagen Implant I | – | Integumental | – | ||
| Evolence (Skillman, NJ, USA) |
Dermicol-P35 | Ribose | Integumental | [2][135][136][137], NCT00929071, NCT00891774 | |
| Mentor Corp. (Santa Barbara, CA, USA) |
Fibrel | – | Integumental | [138][139] | |
| Tissue Science Labs. (Aldershot, UK) |
Permacol | – | Gastrointestinal | [140][141][142][143][144][145][146] | |
| EternoGen, LLC (Columbia, MO, USA) |
RPC Pure Collagen | Ethylenediamine tetraacetic acid | Integumental | [147] | |
| Aspid S.A. de C.V. (Mexico City, Mexico) www.aspidpharma.com, accessed on 14 February 2023 |
Fibroquel | Polyvinylpyrrolidone | Musculoskeletal | [148][149], NCT04517162 | |
| ColBar LifeScience Ltd. (Tel Aviv, Israel) www.ortho-dermatologics.com, accessed on 14 February 2023 |
Evolence | Ribose | Integumental | [135][150] | |
| Human | Fascia Biosystem (Beverly Hills, CA, USA) |
Fascian | Lidocain | Integumental | [6][151][152] |
| Fibrocell Science (Exton, PA, USA) www.fibrocell.com, accessed on 14 February 2023 |
Isolagen therapy | – | Integumental | NCT00655356 | |
| Inamed Corporation (Santa Barbara, CA, USA) www.inamed-cro.com, accessed on 14 February 2023 |
Cosmoplast | Glutaraldehyde, lidocaine hydrochloride | Integumental | [6][153] | |
| Cosmoderm | lidocaine hydrochloride | Integumental | [6][153] | ||
| Life Cell Corp. (Branchburg, NJ, USA) |
Dermalogen | Type and VI collagen, elastin, fibronectin, chondroitin sulfate, and other proteoglycans | Integumental | [154] | |
| Cymetra | Elascin, glycosaminoglycans, Lidocaine hydrochloride | Integumental | [6][96][155][156][157][158] | ||
| Collagenesis, Inc., (Beverly, MA, USA) |
Autologen | Elastin, fibronectin, glycosaminoglycans | Integumental | – | |
| Dermologen | - | Integumental | [156] | ||
| Plant | Vesco Pharmaceutical Co. Ltd. (Bangkok, Thailand) www.vescopharma.com, accessed on 14 February 2023 |
Collagen C 1000 | Vitamin C | Integumental | – |
| Silkworm | Monodermà (Milan, Italy) www.monoderma.com |
Fillagen | Hyaluronic acid, carboxymethylcellulose | Integumental | [159] |
| n. d. | Taumed (Rome, Italy) www.taumed.it, accessed on 14 February 2023 |
Karisma | Hyaluronic acid, carboxymethylcellulose | Integumental | – |
| n. d. | LABO International S.r.l. (Padova, Italy) www.labosuisse.com, accessed on 14 February 2023 |
Fillerina con 3D collagen |
Hyaluronic acid | Integumental | – |
| n. d. | Hebey Mepha Pharm Group Co., Ltd. (Shandong, Hebei, China) www.mephacn.com, accessed on 14 February 2023 |
Collagen Plus | – | Integumental | – |
| n. d. | Pierre Mulot Laboratories (Paris, France) |
Neutroskin | Vitamin C | Integumental | – |
| n. d. | Elements Pharmaceuticals (Shijiazhuang, Hebei, China) www.elementspharma.com, accessed on 14 February 2023 |
Ele-collagen | Vitamin C, Vitamin B6 | Integumental | – |
| n. d. | Globus Medical (Audubon, PA, USA) www.globusmedical.com, accessed on 14 February 2023 |
Kinex Bioactive gel | Bioglass, hyaluronic acid | Musculoskeletal | – |
4. Clinical Efficacy of Collagen-Based Injectable Implants
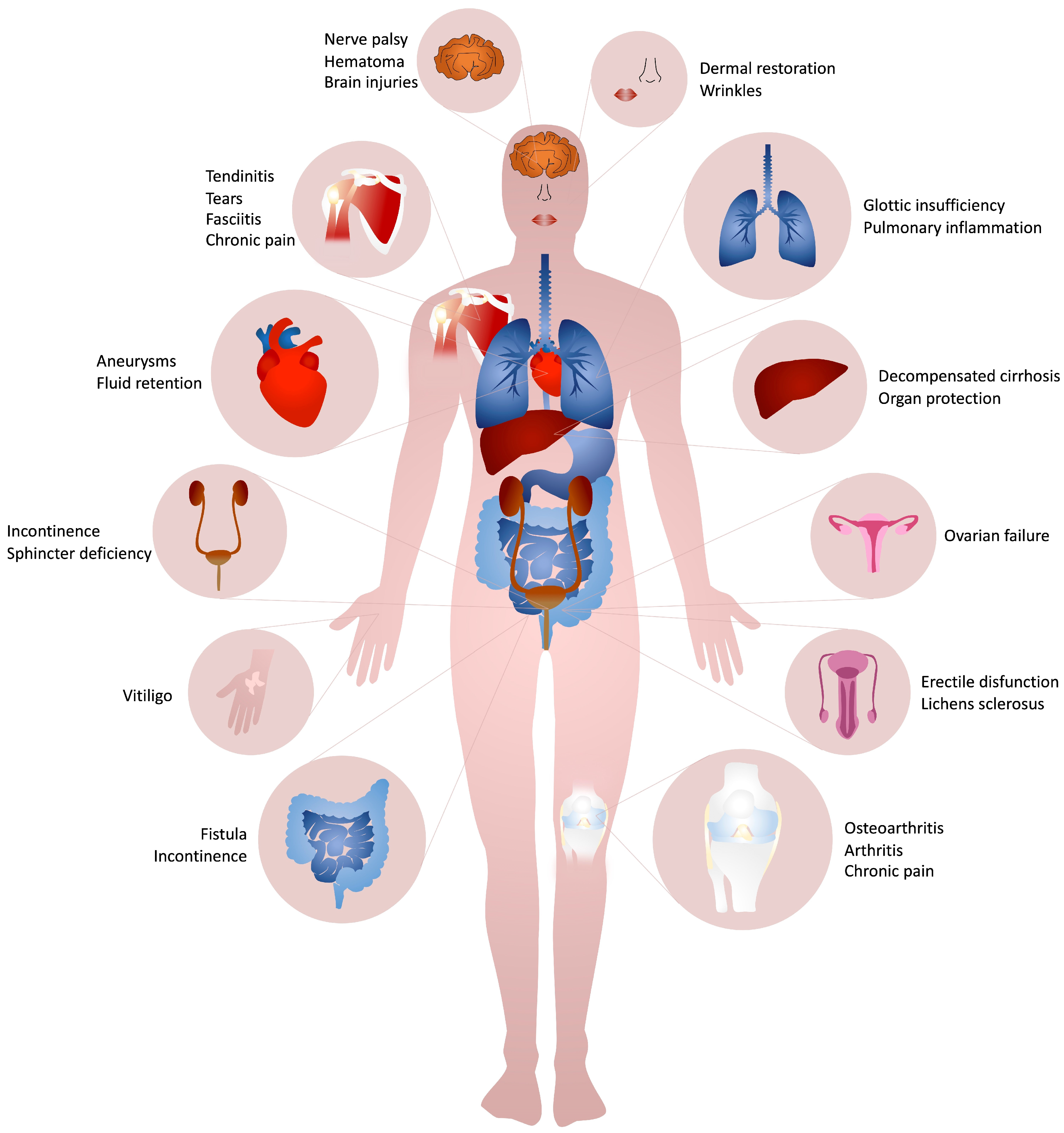
4.1. Integumental Apparatus
4.2. Musculoskeletal Apparatus
4.3. Urogenital System
4.4. Gastrointestinal Apparatus
5. Adverse Reactions to Collagen-Based Injectable Implants
References
- Go, B.C.; Frost, A.S.; Friedman, O. Using Injectable Fillers for Midface Rejuvenation. Plast. Aesthetic Res. 2021, 8, 39.
- Solish, N.J. Assessment of Recovery Time for the Collagen Products Dermicol-P35 27G and 30G. J. Am. Acad. Dermatol. 2010, 62, 824–830.
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.T.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and Clinical Application of Injectable Hydrogels for Musculoskeletal Therapy. Bioeng. Transl. Med. 2022, 7, e10295.
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable Biomaterials for Regenerating Complex Craniofacial Tissues. Adv. Mater. 2009, 21, 3368–3393.
- Béduer, A.; Genta, M.; Kunz, N.; Verheyen, C.; Martins, M.; Brefie-Guth, J.; Braschler, T. Design of an Elastic Porous Injectable Biomaterial for Tissue Regeneration and Volume Retention. Acta Biomater. 2022, 142, 73–84.
- Eppley, B.L.; Dadvand, B. Injectable Soft-Tissue Fillers: Clinical Overview. Plast. Reconstr. Surg. 2006, 118, 98–106.
- Buck, D.W.; Alam, M.; Kim, J.Y.S. Injectable Fillers for Facial Rejuvenation: A Review. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 11–18.
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse Reactions to Injectable Soft Tissue Fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34.
- Luebberding, S.; Alexiades-Armenakas, M. Safety of Dermal Fillers. J. Drugs Dermatol. 2012, 11, 1053–1058.
- Cheng, L.; Sun, X.; Tang, M.; Jin, R.; Cui, W.; Zhang, Y.-G. An Update Review on Recent Skin Fillers. Plast. Aesthetic Res. 2016, 3, 92–99.
- Ginat, D.T.; Schatz, C.J. Imaging Features of Midface Injectable Fillers and Associated Complications. Am. J. Neuroradiol. 2013, 34, 1488–1495.
- Lemperle, G. Injectable Dermal Fillers—Resorbable or Permanent? In Aesthetic Surgery of the Facial Mosaic; Springer: Berlin/Heidelberg, Germany, 2007; pp. 650–664.
- Oranges, C.M.; Brucato, D.; Schaefer, D.J.; Kalbermatten, D.F.; Harder, Y. Complications of Nonpermanent Facial Fillers: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3851.
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable Biomaterials for Translational Medicine. Mater. Today 2019, 28, 81–97.
- Attenello, N.H.; Maas, C.S. Injectable Fillers: Review of Material and Properties. Facial Plast. Surg. 2015, 31, 29–34.
- Cockerham, K.; Hsu, V. Collagen-Based Dermal Fillers: Past, Present, Future. Facial Plast. Surg. 2009, 25, 106–113.
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Avila Rodriguez, M.I.; Rodriguez Barroso, G.L.; Sanchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26.
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79.
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595.
- Sandri, M.; Tampieri, A.; Salvatore, L.; Sannino, A.; Ghiron, J.H.L.; Condorelli, G. Collagen Based Scaffold for Biomedical Applications. J. Biotechnol. 2010, 150, 29.
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22.
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42.
- Chattopadhyay, S.; Raines, R.T. Review Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833.
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651.
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546.
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Parvizi, J.; Kim, G.K. Collagen. In High Yield Orthopaedics; Elsevier: Amsterdam, The Netherlands, 2010; pp. 107–109.
- Goldberga, I.; Li, R.; Duer, M.J. Collagen Structure–Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629.
- Owczarzy, A.; Kurasinski, R.; Kulig, K.; Rogoz, W.; Szkudlarek, A.; Maciazek-Juczyk, M. Collagen—Stucture, Properties and Applications. Eng. Biomater. 2020, 156, 17–23.
- Arseni, L.; Lombardi, A.; Orioli, D. From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci. 2018, 19, 1407.
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868.
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 1–74.
- Shoulder, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307.
- Birk, D.E.; Brückner, P. Collagens, Suprastructures, and Collagen Fibril Assembly. In The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–115.
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An Insight on Type I Collagen from Horse Tendon for the Manufacture of Implantable Devices. Int. J. Biol. Macromol. 2020, 154, 291–306.
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol, E.N. Determination of Hydroxyproline in Tissues and the Evaluation of the Collagen Content of the Tissues. J. Anal. Chem. 2007, 62, 51–57.
- Bou-Gharios, G.; Abraham, D.; de Crombrugghe, B. Type I Collagen Structure, Synthesis, and Regulation. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–337.
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen Fibril Formation. Biochem. J. 1996, 316, 1–11.
- Collins, C.J.; Andriotis, O.G.; Nedelkovski, V.; Frank, M.; Katsamenis, O.L.; Thurner, P.J. Bone Micro- and Nanomechanics. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 22–44.
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; de Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017.
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; de Caro, L.; et al. Investigations of Processing–Induced Structural Changes in Horse Type-I Collagen at Sub and Supramolecular Levels. Front. Bioeng. Biotechnol. 2019, 7, 203.
- von der Mark, K. Structure, Biosynthesis and Gene Regulation of Collagens in Cartilage and Bone. In Dynamics of Bone and Cartilage Metabolism; Elsevier: Amsterdam, The Netherlands, 2006; pp. 3–40.
- Exposito, J.-Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of Collagens. Anat. Rec. 2002, 268, 302–316.
- Chu, M.-L.; de Wet, W.; Bernard, M.; Ding, J.-F.; Morabito, M.; Myers, J.; Williams, C.; Ramirez, F. Human Proα1(I) Collagen Gene Structure Reveals Evolutionary Conservation of a Pattern of Introns and Exons. Nature 1984, 310, 337–340.
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The Triple Helix of Collagens—An Ancient Protein Structure That Enabled Animal Multicellularity and Tissue Evolution. J. Cell Sci. 2018, 131, jcs203950.
- Exposito, J.-Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The Fibrillar Collagen Family. Int. J. Mol. Sci. 2010, 11, 407–426.
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354.
- Ellingsworth, L.R.; de Lustro, F.; Brennan, J.E.; Sawamura, S.; Mc Pherson, J. The Human Immune Response to Reconstituted Bovine Collagen. J. Immunol. 1986, 136, 877–882.
- Charriere, G.; Bejot, M.; Schnitzler, L.; Ville, G.; Hartmann, D.J. Reactions to a Bovine Collagen Implant: Clinical and Immunologic Study in 705 Patients. J. Am. Acad. Dermatol. 1989, 21, 1203–1208.
- Aamodt, J.M.; Grainger, D.W. Extracellular Matrix-Based Biomaterial Scaffolds and the Host Response. Biomaterials 2016, 86, 68–82.
- Lemperle, G.; Morhenn, V.; Charrier, U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plast. Surg. 2003, 27, 354–366.
- Narins, R.S.; Brandt, F.; Leyden, J.; Lorenc, Z.P.; Rubin, M.; Smith, S. A Randomized, Double-Blind, Multicenter Comparison of the Efficacy and Tolerability of Restylane versus Zyplast for the Correction of Nasolabial Folds. Dermatol. Surg. 2003, 29, 588–595.
- Solomon, P.; Sklar, M.; Zener, R. Facial Soft Tissue Augmentation with Artecoll®: A Review of Eight Years of Clinical Experience in 153 Patients. Can. J. Plast. Surg. 2012, 20, 28–32.
- Lemperle, G.; Romani, J.J.; Busso, M. Soft Tissue Augmentation With Artecoll: 10-Year History, Indications, Techniques, and Complications. Dermatol. Surg. 2003, 29, 573–587.
- Cohen, S.R.; Holmes, R.E. Artecoll: A Long-Lasting Injectable Wrinkle Filler Material: Report of a Controlled, Randomized, Multicenter Clinical Trial of 251 Subjects. Plast. Reconstr. Surg. 2004, 114, 964–976.
- Haneke, E. Polymethyl Methacrylate Microspheres in Collagen. Semin. Cutan. Med. Surg. 2004, 23, 227–232.
- Kim, K.J.; Lee, H.W.; Lee, M.W.; Choi, J.H.; Moon, K.C.; Koh, J.K. Artecoll Granuloma: A Rare Adverse Reaction Induced by Microimplant in the Treatment of Neck Wrinkles. Dermatol. Surg. 2004, 30, 545–547.
- Rullan, P.P. Soft Tissue Augmentation Using Artecoll: A Personal Experience. Facial Plast. Surg. 2004, 20, 111–116.
- Thaler, M.P.; Ubogy, Z.I. Artecoll: The Arizona Experience and Lessons Learned. Dermatol. Surg. 2005, 31, 1566–1576.
- Solomon, P.; Ng, C.L.; Kerzner, J.; Rival, R. Facial Soft Tissue Augmentation with Bellafill: A Review of 4 Years of Clinical Experience in 212 Patients. Plast. Surg. 2021, 29, 98–102.
- Cohen, S.R.; Berner, C.F.; Busso, M.; Gleason, M.C.; Hamilton, D.; Holmes, R.E.; Romano, J.J.; Rullan, P.P.; Thaler, M.P.; Ubogy, Z.; et al. ArteFill: A Long-Lasting Injectable Wrinkle Filler Material—Summary of the U.S. Food and Drug Administration Trials and a Progress Report on 4- to 5-Year Outcomes. Plast. Reconstr. Surg. 2006, 118, 64–76.
- Moon, S.H.; Lee, Y.J.; Rhie, J.W.; Suh, D.S.; Oh, D.Y.; Lee, J.H.; Kim, Y.J.; Kim, S.M.; Jun, Y.J. Comparative Study of the Effectiveness and Safety of Porcine and Bovine Atelocollagen in Asian Nasolabial Fold Correction. J. Plast. Surg. Hand Surg. 2015, 49, 147–152.
- Lee, J.H.; Choi, Y.S.; Kim, S.M.; Kim, Y.J.; Rhie, J.W.; Jun, Y.J. Efficacy and Safety of Porcine Collagen Filler for Nasolabial Fold Correction in Asians: A Prospective Multicenter, 12 Months Follow-up Study. J. Korean Med. Sci. 2014, 29, S217–S221.
- Hirche, C.; Senghaas, A.; Fischer, S.; Hollenbeck, S.T.; Kremer, T.; Kneser, U. Novel Use of a Flowable Collagen-Glycosaminoglycan Matrix (IntegraTM Flowable Wound Matrix) Combined with Percutaneous Cannula Scar Tissue Release in Treatment of Post-Burn Malfunction of the Hand—A Preliminary 6 Month Follow-Up. Burns 2016, 42, e1–e7.
- Kligman, A.M.; Armstrong, R.C. Histologic Respose to Intradermal Zyderm and Zyplast (Glutaraldehyde Cross-Linked) Collagen in Humans. J. Dermatol. Surg. Oncol. 1986, 12, 351–357.
- Stegman, S.J.; Chu, S.; Bensch, K.; Armstrong, R. A Light and Electron Microscopic Evaluation of Zyderm Collagen and Zyplast Implants in Aging Human Facial Skin: A Pilot Study. Arch. Dermatol. 1987, 123, 1644–1649.
- Elson, M.L. Clinical Assessment of Zyplast Implant: A Year of Experience for Soft Tissue Contour Correction. J. Am. Acad. Dermatol. 1988, 18, 707–713.
- Matti, B.A.; Nicolle, F.v. Clinical Use of Zyplast in Correction of Age- and Disease-Related Contour Deficiencies of the Face. Aesthetic Plast. Surg. 1990, 14, 227–234.
- Cooperman, L.S.; Mackinnon, V.; Bechler, G.; Pharriss, B.B. Injectable Collagen: A Six-Year Clinical Investigation. Aesthetic Plast. Surg. 1985, 9, 145–151.
- Castrow, F.F.; Krull, E.A. Injectable Collagen Implant—Update. J. Am. Acad. Dermatol. 1983, 9, 889–893.
- Downie, J.; Mao, Z.; Rachel Lo, T.W.; Barry, S.; Bock, M.; Siebert, J.P.; Bowman, A.; Ayoub, A. A Double-Blind, Clinical Evaluation of Facial Augmentation Treatments: A Comparison of PRI 1, PRI 2, Zyplast® and Perlane®. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1636–1643.
- Baumann, L.S.; Shamban, A.T.; Lupo, M.P.; Monheit, G.D.; Thomas, J.A.; Murphy, D.K.; Walker, P.S. Comparison of Smooth-Gel Hyaluronic Acid Dermal Fillers with Cross-Linked Bovine Collagen: A Multicenter, Double-Masked, Randomized, within-Subject Study. Dermatol. Surg. 2007, 33, 128–135.
- Nevins, M.; Giannobile, W.V.; McGuire, M.K.; Kao, R.T.; Mellonig, J.T.; Hinrichs, J.E.; McAllister, B.S.; Murphy, K.S.; McClain, P.K.; Nevins, M.L.; et al. Platelet-Derived Growth Factor Stimulates Bone Fill and Rate of Attachment Level Gain: Results of a Large Multicenter Randomized Controlled Trial. J. Periodontol. 2005, 76, 2205–2215.
- Nevins, A.; Crespi, P. A Clinical Study Using the Collagen Gel Zyplast in Endodontic Treatment. J. Endod. 1998, 24, 610–613.
- Gadomski, B.C.; Labus, K.M.; Puttlitz, C.M.; McGilvray, K.C.; Regan, D.P.; Nelson, B.; Seim, H.B.; Easley, J.T. Evaluation of Lumbar Spinal Fusion Utilizing Recombinant Human Platelet Derived Growth Factor-B Chain Homodimer (RhPDGF-BB) Combined with a Bovine Collagen/β-Tricalcium Phosphate (β-TCP) Matrix in an Ovine Model. JOR Spine 2021, 4, e1166.
- Scott, R.T.; McAlister, J.E.; Rigby, R.B. Allograft Bone: What Is the Role of Platelet-Derived Growth Factor in Hindfoot and Ankle Fusions. Clin. Podiatr. Med. Surg. 2018, 35, 37–52.
- Daniels, T.R.; Anderson, J.; Swords, M.P.; Maislin, G.; Donahue, R.; Pinsker, E.; Quiton, J.D. Recombinant Human Platelet–Derived Growth Factor BB in Combination with a Beta-Tricalcium Phosphate (RhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019, 40, 1068–1078.
- DiGiovanni, C.W.; Lin, S.S.; Baumhauer, J.F.; Daniels, T.; Younger, A.; Glazebrook, M.; Anderson, J.; Anderson, R.; Evangelista, P.; Lynch, S.E.; et al. Recombinant Human Platelet-Derived Growth Factor-BB and Beta-Tricalcium Phosphate (RhPDGF-BB/β-TCP): An Alternative to Autogenous Bone Graft. J. Bone Jt. Surg. 2013, 95, 1184–1192.
- Daniels, T.; DiGiovanni, C.; Lau, J.T.C.; Wing, K.; Alastair, Y. Prospective Clinical Pilot Trial in a Single Cohort Group of RhPDGF in Foot Arthrodeses. Foot Ankle Int. 2010, 31, 473–479.
- Abidi, N.A.; Younger, A.; Digiovanni, C.W. Role of Platelet-Derived Growth Factor in Hindfoot Fusion. Tech. Foot Ankle Surg. 2012, 11, 34–38.
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant Human Platelet-Derived Growth Factor: Biology and Clinical Applications. J. Bone Jt. Surg. 2008, 90, 48–54.
- DiGiovanni, C.W.; Petricek, J.M. The Evolution of RhPDGF-BB in Musculoskeletal Repair and Its Role in Foot and Ankle Fusion Surgery. Foot Ankle Clin. 2010, 15, 621–640.
- Digiovanni, C.W.; Lin, S.; Pinzur, M. Recombinant Human PDGF-BB in Foot and Ankle Fusion. Expert Rev. Med. Devices 2012, 9, 111–122.
- DiGiovanni, C.W.; Baumhauer, J.; Lin, S.S.; Berberian, W.S.; Flemister, A.S.; Enna, M.J.; Evangelista, P.; Newman, J. Prospective, Randomized, Multi-Center Feasibility Trial of RhPDGF-BB versus Autologous Bone Graft in a Foot and Ankle Fusion Model. Foot Ankle Int. 2011, 32, 344–354.
- DiGiovanni, C.W.; Lin, S.S.; Daniels, T.R.; Glazebrook, M.; Evangelista, P.; Donahue, R.; Beasley, W.; Baumhauer, J.F. The Importance of Sufficient Graft Material in Achieving Foot or Ankle Fusion. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1260–1267.
- Solchaga, L.A.; Daniels, T.; Roach, S.; Beasley, W.; Snel, L.B. Effect of Implantation of Augment® Bone Graft on Serum Concentrations of Platelet-Derived Growth Factors: A Pharmacokinetic Study. Clin. Drug Investig. 2013, 33, 143–149.
- Perrien, D.S.; Young, C.S.; Alvarez-Urena, P.P.; Dean, D.D.; Lynch, S.E.; Hollinger, J.O. Percutaneous Injection of Augment Injectable Bone Graft (RhPDGF-BB and β-Tricalcium Phosphate /Bovine Type i Collagen Matrix) Increases Vertebral Bone Mineral Density in Geriatric Female Baboons. Spine J. 2013, 13, 580–586.
- de Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; de Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional in Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975.
- Kimura, M.; Nito, T.; Imagawa, H.; Tayama, N.; Chan, R.W. Collagen Injection as a Supplement to Arytenoid Adduction for Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2008, 117, 430–436.
- Kimura, M.; Nito, T.; Sakakibara, K.I.; Tayama, N.; Niimi, S. Clinical Experience with Collagen Injection of the Vocal Fold: A Study of 155 Patients. Auris Nasus Larynx 2008, 35, 67–75.
- Stojkovic, S.G.; Lim, M.; Burke, D.; Finan, P.J.; Sagar, P.M. Intra-Anal Collagen Injection for the Treatment of Faecal Incontinence. Br. J. Surg. 2006, 93, 1514–1518.
- Jamal, N.; Mundi, J.; Chhetri, D.K. Higher Risk of Superficial Injection during Injection Laryngoplasty in Women. Am. J. Otolaryngol. 2014, 35, 159–163.
- Ford, C.N.; Bless, D.M.; Loftus, J.M. Role of Injectable Collagen in the Treatment of Glottic Insufficiency: A Study of 119 Patients. Ann. Otol. Rhinol. Laryngol. 1992, 101, 237–247.
- Hoffman, H.; McCabe, D.; McCulloch, T.; Jin, S.M.; Karnell, M. Laryngeal Collagen Injection as an Adjunct to Medialization Laryngoplasty. Laryngoscope 2002, 112, 1407–1413.
- Luu, Q.; Tsai, V.; Mangunta, V.; Berke, G.S.; Chhetri, D.K. Safety of Percutaneous Injection of Bovine Dermal Crosslinked Collagen for Glottic Insufficiency. Otolaryngol. Head Neck Surg. 2007, 136, 445–449.
- Kamer, F.M.; Churukian, M.M. Clinical Use of Injectable Collagen. Arch. Otolaryngol. 1984, 110, 93–98.
- Cespedes, R.D. Collagen Injection or Artificial Sphincter for Postprostatectomy Incontinence: Collagen. Urology 2000, 55, 5–7.
- Homma, Y.; Kawabe, K.; Kageyama, S.; Koiso, K.; Akaza, H.; Kakizoe, T.; Koshiba, K.; Yokoyama, E.; Aso, Y. Injection of Glutaraldehyde Cross-Linked Collagen for Urinary Incontinence: Two-Year Efficacy by Self-Assessment. Int. J. Urol. 1996, 3, 124–127.
- Elsergany, R.; Elgamasy, A.N.; Ghoniem, G.M. Transurethral Collagen Injection for Female Stress Incontinence. Int. Urogynecol. J. 1998, 9, 13–18.
- Cespedes, R.D.; Leng, W.W.; McGuire, E.J. Collagen Injection Therapy for Postprostatectomy Incontinence. Urology 1999, 54, 597–602.
- Corcos, J.; Fournier, C. Periurethral Collagen Injection for the Treatment of Female Stress Urinary Incontinence: 4-Year Follow-up Results. Urology 1999, 54, 815–818.
- Gorton, E.; Stanton, S.; Monga, A.; Wiskind, A.K.; Lentz, G.M.; Bland, D.R. Periurethral Collagen Injection: A Long-Term Follow-up Study. BJU Int. 1999, 84, 966–971.
- Vandenbulcke, L.; Lapage, K.G.; Vanderstraeten, K.V.; de Somer, F.M.; de Hert, S.G.; Moerman, A.T. Microvascular Reactivity Monitored with Near-Infrared Spectroscopy Is Impaired after Induction of Anaesthesia in Cardiac Surgery Patients. Eur. J. Anaesthesiol. 2017, 34, 688–694.
- Marx, G.; Zacharowski, K.; Ichai, C.; Asehnoune, K.; Černý, V.; Dembinski, R.; Ferrer Roca, R.; Fries, D.; Molnar, Z.; Rosenberger, P.; et al. Efficacy and Safety of Early Target-Controlled Plasma Volume Replacement with a Balanced Gelatine Solution versus a Balanced Electrolyte Solution in Patients with Severe Sepsis/Septic Shock: Study Protocol, Design, and Rationale of a Prospective, Randomized, Controlled, Double-Blind, Multicentric, International Clinical Trial: GENIUS—Gelatine Use in ICU and Sepsis. Trials 2021, 22, 1–12.
- Hamraoui, K.; Ernst, S.M.P.G.; van Dessel, P.F.H.M.; Kelder, J.C.; ten Berg, J.M.; Suttorp, J.; Jaarsma, W.; Plokker, H.W. Efficacy and Safety of Percutaneous Treatment of Iatrogenic Femoral Artery Pseudoaneurysm by Biodegradable Collagen Injection. J. Am. Coll. Cardiol. 2002, 39, 1297–1304.
- Majdalany, B.S.; Willatt, J.; Beecham Chick, J.F.; Srinivasa, R.N.; Saad, W.A. Fibrillar Collagen Injection for Organ Protection during Thermal Ablation of Hepatic Malignancies. Diagn. Interv. Radiol. 2017, 23, 381–384.
- Sparavigna, A.; Tateo, A.; Inselvini, E.; Tocchio, M.; Orlandini, M.C.; Botali, G. Anti-Age Activity and Tolerance Evaluation of Collagen Micro-Injection Treatment Associated to Topical Application of a Cosmetic Formulation (Investigator-Initiated Multicentre Trial). J. Clin. Exp. Dermatol. Res. 2017, 8, 1000391.
- Gkouvi, A.; Nicolaidou, E.; Corbo, A.; Selvaggi, G.; Tsimpidakis, A.; Mastraftsi, S.; Gregoriou, S. Heterologous Type i Collagen as an Add-on Therapy to Narrowband Ultraviolet b for the Treatment of Vitiligo: A Pilot Study. J. Clin. Aesthetic Dermatol. 2021, 14, 31–34.
- Gkouvi, A.; Corbo, A.; Gregoriou, S. Treatment of Male Genital Lichen Sclerosus with Heterologous Type I Collagen. Clin. Exp. Dermatol. 2020, 45, 388–390.
- Klewin-Steinböck, S.; Nowak-Terpi3owska, A.; Adamski, Z.; Grocholewicz, K.; Wyganowska-Swiatkowska, M. Effect of Injectable Equine Collagen Type I on Metabolic Activity and Apoptosis of Gingival Fibroblasts. Adv. Dermatol. Allergol. 2021, XXXVIII, 440–445.
- Wyganowska-Swiatkowska, M.; Duda-Sobczak, A.; Corbo, A.; Matthews-Brzozowska, T. Atelocollagen Application in Human Periodontal Tissue Treatment—A Pilot Study. Life 2020, 10, 114.
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Giusti, I.; Dolo, V.; Guerrini, L.; Cifone, M.G.; Giuliani, M.; Cinque, B. Type I Collagen Suspension Induces Neocollagenesis and Myodifferentiation in Fibroblasts in Vitro. Biomed. Res. Int. 2020, 2020, 6093974.
- Maternini, M.; Guttadauro, A.; Mascagni, D.; Milito, G.; Stuto, A.; Renzi, A.; Ripamonti, L.; Bottini, C.; Nudo, R.; del Re, L.; et al. Non Cross-Linked Equine Collagen (Salvecoll-E Gel) for Treatment of Complex Ano-Rectal Fistula. Asian J. Surg. 2019, 43, 401–404.
- Keefe, J.; Wauk, L.; Chu, S.; DeLustro, F. Clinical Use of Injectable Bovine Collan: A Decade of OExperience. Clin. Mater. 1992, 9, 155–162.
- Randelli, F.; Menon, A.; Via, A.G.; Mazzoleni, M.; Sciancalepore, F.; Brioschi, M.; Gagliano, N. Effect of a Collagen-Based Compound on Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2018, 7, 246.
- Nitecka-Buchta, A.; Walczynska-Dragon, K.; Batko-Kapustecka, J.; Wieckiewicz, M. Comparison between Collagen and Lidocaine Intramuscular Injections in Terms of Their Efficiency in Decreasing Myofascial Pain within Masseter Muscles: A Randomized, Single-Blind Controlled Trial. Pain Res. Manag. 2018, 2018, 8261090.
- Giovannangeli, F.; Bizzi, E.; Massafra, U.; Vacca, F.; Tormenta, S.; Migliore, A. Intra-Articular Administration of MD-HIP in 24 Patients Affected by Symptomatic Hip Osteoarthritis—A 24-Month Cohort Study. Physiol. Regul. Med. 2016, 31–32.
- Guitart Vela, J.; Folch Ibanez, J. Collagen MDs for Chronic Pain. Efficacy and Tolerability in Chronic Treatment in 124 Patients. Physiol. Regul. Med. 2016, 9–12.
- Reshkova, V.; Rashkov, R.; Nestorova, R. Efficacy and Safety Evaluation of GUNA Collagen MDs Injections in Knee Osteoarthritis—A Case Series of 30 Patients. Physiol. Regul. Med. 2016, 27–29.
- Mariconti, P. Usefulness of GUNA Collagen Medical Devices in the Treatment of Knee Pain. Physiol. Regul. Med. 2016, 39–40.
- Martin Martin, L.S.; Massafra, U.; Bizzi, E.; Migliore, A. A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint”). BMC Musculoskelet. Disord. 2016, 17, 1–8.
- Pavelka, K.; Jarosova, H.; Milani, L.; Prochazka, Z.; Kostiuk, P.; Kotlarova, L.; Meroni, A.M.; Slíva, J. Efficacy and Tolerability of Injectable Collagen-Containing Products in Comparison to Trimecaine in Patients with Acute Lumbar Spine Pain (Study FUTURE-MD-Back Pain). Physiol. Res. 2019, 68, s65–s74.
- Massulo, C. Injectable GUNA Collagen Medical Devices in Functional Recovery from Sport. Physiol. Regul. Med. 2016, 3–7.
- Uroz, N.Z. Collagen Medical Device Infiltrations in Shoulder Pathologies. Calcific Supraspinatus Tendinitis. Physiol. Regul. Med. 2016, 15–17.
- Nestorova, R.; Rashkov, R.; Petranova, T. Clinical and Sonographic Assessment of the Effectiveness of GUNA Collagen MDs Injections in Patients with Partial Thickness Tear of the Rotator Cuff. Physiol. Regul. Med. 2016, 35–37.
- Staňa, J. 3 Years in Luhačovice Spa with Collagen Medical Devices Injections in the Treatment of Piriformis Syndrome. Physiol. Regul. Med. 2016, 19–20.
- Micarelli, A.; Viziano, A.; Granito, I.; Antonuccio, G.; Felicioni, A.; Loberti, M.; Carlino, P.; Micarelli, R.X.; Alessandrini, M. Combination of In-Situ Collagen Injection and Rehabilitative Treatment in Long-Lasting Facial Nerve Palsy: A Pilot Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 366–375.
- Alfieri, N. MD-Muscle in the Management of Myofascial Pain Syndrome. Physiol. Regul. Med. 2016, 23–24.
- Heng, C.H.Y.; Snow, M.; Dave, L.Y.H. Single-Stage Arthroscopic Cartilage Repair With Injectable Scaffold and BMAC. Arthrosc. Tech. 2021, 10, e751–e756.
- Kim, M.S.; Chun, C.H.; Wang, J.H.; Kim, J.G.; Kang, S.B.; Yoo, J.D.; Chon, J.G.; Kim, M.K.; Moon, C.W.; Chang, C.B.; et al. Microfractures Versus a Porcine-Derived Collagen-Augmented Chondrogenesis Technique for Treating Knee Cartilage Defects: A Multicenter Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 1612–1624.
- Lee, H.S.; Oh, K.J.; Moon, Y.W.; In, Y.; Lee, H.J.; Kwon, S.Y. Intra-Articular Injection of Type I Atelocollagen to Alleviate Knee Pain: A Double-Blind, Randomized Controlled Trial. Cartilage 2021, 13, 342S–350S.
- Kim, J.H.; Kim, D.J.; Lee, H.J.; Kim, B.K.; Kim, Y.S. Atelocollagen Injection Improves Tendon Integrity in Partial-Thickness Rotator Cuff Tears: A Prospective Comparative Study. Orthop. J. Sports Med. 2020, 8, 2325967120904012.
- Wolkow, N.; Jakobiec, F.A.; Dryja, T.P.; Lefebvre, D.R. Mild Complications or Unusual Persistence of Porcine Collagen and Hyaluronic Acid Gel Following Periocular Filler Injections. Ophthalmic Plast. Reconstr. Surg. 2018, 34, e143–e146.
- Braun, M.; Braun, S. Nodule Formation Following Lip Augmentation Using Porcine Collagen-Derived Filler. J. Drugs Dermatol. 2008, 7, 579–581.
- Narins, R.S.; Brandt, F.S.; Lorenc, Z.P.; Maas, C.S.; Monheit, G.D.; Smith, S.R. Twelve-Month Persistency of a Novel Ribose-Cross-Linked Collagen Dermal Filler. Dermatol. Surg. 2008, 34, 31–39.
- Pollack, S.v. Silicone, Fibrel, and Collagen Implantation for Facial Lines and Wrinkles. J. Dermatol. Surg. Oncol. 1990, 16, 957–961.
- Mittelman, H. Fibrel: A Dermal Implant Comparison With Collagen Implants. Arch. Otolaryngol. Head Neck Surg. 1988, 114, 1379.
- Samalavicius, N.E.; Kavaliauskas, P.; Dulskas, A. PermacolTM Collagen Paste Injection for the Treatment of Complex Anal Fistula—A Video Vignette. Color. Dis. 2020, 22, 116–117.
- Pescatori, M. PermacolTM Collagen Paste for Treating a Rectovaginal Fistula Following Anterior Rectal Prolapsectomy. Tech. Coloproctol. 2017, 21, 909–910.
- Harran, N.; Herold, J.; Bentley, A.; Bebington, B.D. Efficacy of Porcine Dermal Collagen (PermacolTM) Injection for Passive Faecal Incontinence in a Dedicated Colorectal Unit at the Wits Donald Gordon Medical Centre. S. Afr. J. Surg. 2017, 55, 10–13.
- Giordano, P.; Sileri, P.; Buntzen, S.; Stuto, A.; Nunoo-Mensah, J.; Lenisa, L.; Singh, B.; Thorlacius-Ussing, O.; Griffiths, B.; Ziyaie, D. A Prospective Multicentre Observational Study of PermacolTM Collagen Paste for Anorectal Fistula: Preliminary Results. Color. Dis. 2016, 18, 286–294.
- Sileri, P.; Franceschilli, L.; del Vecchio Blanco, G.; Stolfi, V.M.; Angelucci, G.P.; Gaspari, A.L. Porcine Dermal Collagen Matrix Injection May Enhance Flap Repair Surgery for Complex Anal Fistula. Int. J. Color. Dis. 2011, 26, 345–349.
- Milito, G.; Cadeddu, F. Conservative Treatment for Anal Fistula: Collagen Matrix Injection. J. Am. Coll. Surg. 2009, 209, 542–543.
- Harper, C. Permacol: Clinical Experience with a New Biomaterial. Hosp. Med. 2001, 62, 90–95.
- Inglefield, C.; Samuelson, E.U.; Landau, M.; DeVore, D. Bio-Dermal Restoration With Rapidly Polymerizing Collagen: A Multicenter Clinical Study. Aesthetic Surg. J. 2018, 38, 1131–1138.
- del Carpio-Orantes, L.; García-Méndez, S.; Sánchez-Díaz, J.S.; Aguilar-Silva, A.; Contreras-Sánchez, E.R.; Hernández, S.N.H. Use of Fibroquel® (Polymerized Type I Collagen) in Patients with Hypoxemic Inflammatory Pneumonia Secondary to COVID-19 in Veracruz, Mexico. J. Anesth. Crit. Care 2021, 13, 69–73.
- Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; Ochoa-Hein, E.; Perez-Ortiz, A.; Rendón-Macías, M.E.; Rojas-Castañeda, E.; Urbina-Terán, S.; et al. Effect of Polymerised Type I Collagen on Hyperinflammation of Adult Outpatients with Symptomatic COVID-19. Clin. Transl. Med. 2022, 12, 1–8.
- Denton, A.B.; Shoman, N. Porcine Collagen: Evolence. In Office-Based Cosmetic Procedures and Techniques; Cambridge University Press: Cambridge, UK, 2010; pp. 68–70.
- Burres, S. Soft-Tissue Augmentation with Fascian. Clin. Plast. Surg. 2001, 28, 101–110.
- Burres, S. Fascian. Facial Plast. Surg. 2004, 20, 149–152.
- Bauman, L. CosmoDerm/CosmoPlast (Human Bioengineered Collagen) for the Aging Face. Facial Plast. Surg. 2004, 20, 125–128.
- Fagien, S.; Elson, M.L. Facial Soft-Tissue Augmentation with Allogeneic Human Tissue Collagen Matrix (Dermalogen and Dermaplant). Clin. Plast. Surg. 2001, 28, 63–81.
- Maloney, B.P.; Murphy, B.A.; Cole, H.P. Cymetra. Facial Plast. Surg. 2004, 20, 129–134.
- Anderson, T.D.; Sataloff, R.T. Complications of Collagen Injection of the Vocal Fold: Report of Several Unusual Cases and Review of the Literature. J. Voice 2004, 18, 392–397.
- Bock, J.M.; Lee, J.H.; Robinson, R.A.; Hoffman, H.T. Migration of Cymetra After Vocal Fold Injection for Laryngeal Paralysis. Laryngoscope 2007, 117, 2251–2254.
- Karpenko, A.N.; Meleca, R.J.; Dworkin, J.P.; Stachler, R.J. Cymetra Injection for Unilateral Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2003, 112, 927–934.
- Rinaldi, F.; Pinto, D.; Trink, A.; Giuliani, G.; Sparavigna, A. In Vitro and in Vivo Evaluation on the Safety and Efficacy of a Brand-New Intracutaneous Filler with A1-R-Collagen. Clin. Cosmet. Investig. Dermatol. 2021, 14, 501–512.
- Volpi, P.; Zini, R.; Erschbaumer, F.; Beggio, M.; Busilacchi, A.; Carimati, G. Effectiveness of a Novel Hydrolyzed Collagen Formulation in Treating Patients with Symptomatic Knee Osteoarthritis: A Multicentric Retrospective Clinical Study. Int. Orthop. 2021, 45, 375–380.
- Furuzawa-Carballeda, J.; Lima, G.; Llorente, L.; Nuñez-Álvarez, C.; Ruiz-Ordaz, B.H.; Echevarría-Zuno, S.; Hernández-Cuevas, V. Polymerized-Type I Collagen Downregulates Inflammation and Improves Clinical Outcomes in Patients with Symptomatic Knee Osteoarthritis Following Arthroscopic Lavage: A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Sci. World J. 2012, 2012, 342854.
- Corrado, B.; Bonini, I.; Alessio Chirico, V.; Rosano, N.; Gisonni, P. Use of Injectable Collagen in Partial-Thickness Tears of the Supraspinatus Tendon: A Case Report. Oxf. Med. Case Rep. 2020, 2020, 408–410.
- Kim, M.; Choi, Y.S.; You, M.-W.; Kim, J.S.; Young, K.W. Sonoelastography in the Evaluation of Plantar Fasciitis Treatment 3-Month Follow-Up After Collagen Injection. Ultrasound Q. 2016, 32, 327–332.
- Martins, S.B.; Oliveira, E.; Castro, R.A.; Sartori, M.G.; Baracat, E.C.; Lima, G.R.; Girao, M.J. Clinical and Urodynamic Evaluation in Women with Stress Urinary Incontinence Treated by Periurethral Collagen Injection. Int. Braz. J. Urol. 2007, 33, 695–703.
- Oremus, M.; Tarride, J.-E. An Economic Evaluation of Surgery versus Collagen Injection for the Treatment of Female Stress Urinary Incontinence. Can. J. Urol. 2010, 17, 5087–5093.
- Winters, J.C.; Chiverton, A.; Scarpero, H.M.; Prats, L.J. Collagen injection therapy in elderly women: Long-term results and patient satisfaction. Urology 2000, 55, 856–860.
- Groutz, A.; Blaivas, J.G.; Kesler, S.S.; Weiss, J.P.; Chaikin, D.C. Female urology outcome results of transurethral collagen injection for female stress incontinence: Assessment by urinary incontinence score. J. Urol. 2000, 164, 2006–2009.
- Bomalaski, M.D.; Bloom, D.A.; McGuire, E.J.; Panzl, A. Glutaraldehyde cross-linked collagen in the treatment of urinary incontinence in children. J. Urol. 1996, 155, 699–702.
- Kassouf, W.; Capolicchio, G.; Berardinucci, G.; Corcos, J. Collagen injection for treatment of urinary incontinence in children. J. Urol. 2001, 165, 1666–1668.
- Faerber, G.J.; Richardson, T.D. Long-Term Results of Transurethral Collagen Injection in Men with Intrinsic Sphincter Deficiency; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 1997; Volume 11.
- Faerber, G.J.; Belville, W.D.; Ohl, D.A.; Plata, A. Comparison of Transurethral versus Periurethral Collagen Injection in Women with Intrinsic Sphincter Deficiency. Tech. Urol. 1998, 4, 124–127.
- Richardson, T.D.; Kennelly, M.J.; Faerber, G.J. Endoscopic injection of clutaraldehyde cross-linked collagen for the treatment of intrinsic sphincter deficiency in women. Urology 1995, 43, 378–381.
- Smith, D.N.; Appell, R.A.; Rackley, R.R.; Winters, J.C. Collagen Injection Therapy for Post-Prostatectomy Incontinence. J. Urol. 1998, 160, 364–367.
- Klutke, C.G.; Tiemann, D.D.; Nadler, R.B.; Andriole, G.L. Antegrade Collagen Injection for Stress Incontinence after Radical Prostatectomy: Technique and Early Results; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 1996; Volume 10.
- Appell, R.A. Collagen Injection Therapy for Urinary Incontinence. Urol. Clin. N. Am. 1994, 21, 177–182.
- Appell, R.A.; Vasavada, S.P.; Rackley, R.R.; Winters, J.C. Percutaneous antegrade collagen injection therapy for urinary incontinence following radical prostatectomy. Urology 1996, 48, 769–772.
- Nagai, A.; Nasu, Y.; Watanabe, M.; Tsugawa, M.; Iguchi, H.; Kumon, H. Analysis of Retrograde Ejaculation Using Color Doppler Ultrasonography before and after Transurethral Collagen Injection. Int. J. Impot. Res. 2004, 16, 456–458.
- Spiegel, J.R.; Sataloff, R.T.; Gould, W.J. The Treatment of Vocal Fold Paralysis with Injectable Collagen: Clinical Concerns. J. Voice 1987, 1, 119–121.
- Remacle, M.; Lawson, G. Results with Collagen Injection into the Vocal Folds for Medialization. Curr. Opin. Otolaryngol. Head Neck Surg 2007, 15, 148–152.
- Remacle, M.; Hamoir, M.; Marbaix, E. Gax-Collagen Injection to Correct Aspiration Problems after Subtotal Laryngectomy. Laryngoscope 1990, 100, 662–669.
- Ford, C.N.; Bless, D.M. Selected Problems Treated by Vocal Fold Injection of Collagen. Am. J. Otolaryngol. 1993, 14, 257–261.
- Lundy, D.S.; Casiano, R.R.; McClinton, M.E.; Xue, J.W. Early Results of Transcutaneous Injection Laryngoplasty with Micronized Acellular Dermis Versus Type-I Thyroplasty for Glottic Incompetence Dysphonia Due to Unilateral Vocal Fold Paralysis. J. Voice 2003, 17, 589–595.
- Camilleri-Brennan, J. Anal Injectables and Implantables for Faecal Incontinence. In Fecal Incontinence—Causes, Management and Outcome; InTech: Vienna, Austria, 2014.
- Rydningen, M.; Dehli, T.; Wilsgaard, T.; Rydning, A.; Kumle, M.; Lindsetmo, R.O.; Norderval, S. Sacral Neuromodulation Compared with Injection of Bulking Agents for Faecal Incontinence Following Obstetric Anal Sphincter Injury—A Randomized Controlled Trial. Color. Dis. 2017, 19, O134–O144.
- Brown, S.A.; Rohrich, R.J.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Kenkel, J.M.; Lowe, N.J.; Monheit, G.D.; Narins, R.S.; et al. Subject Global Evaluation and Subject Satisfaction Using Injectable Poly-l-Lactic Acid versus Human Collagen for the Correction of Nasolabial Fold Wrinkles. Plast. Reconstr. Surg. 2011, 127, 1684–1692.
- Narins, R.S.; Baumann, L.; Brandt, F.S.; Fagien, S.; Glazer, S.; Lowe, N.J.; Monheit, G.D.; Rendon, M.I.; Rohrich, R.J.; Werschler, W.P. A Randomized Study of the Efficacy and Safety of Injectable Poly-L-Lactic Acid versus Human-Based Collagen Implant in the Treatment of Nasolabial Fold Wrinkles. J. Am. Acad. Dermatol. 2010, 62, 448–462.
- Draelos, Z. Case Study of Dermicol-P35 Used in Patient with Past Hypersensitivity to Crosslinked Bovine Collagen Dermal Filler. Dermatol. Surg. 2010, 36, 825–827.
- Narins, R.S.; Brandt, F.S.; Lorenc, Z.P.; Maas, C.S.; Monheit, G.D.; Smith, S.R.; Mcintyre, S. A Randomized, Multicenter Study of the Safety and Efficacy of Dermicol-P35 and Non-Animal-Stabilized Hyaluronic Acid Gel for the Correction of Nasolabial Folds. Dermatol. Surg. 2007, 33, S213–S221.
- Cassuto, D. The Use of Dermicol-P35 Dermal Filler for Nonsurgical Rhinoplasty. Aesthetic Surg. J. 2009, 29, S22–S24.
- Smith, K.C. Repair of Acne Scars With Dermicol-P35. Aesthetic Surg. J. 2009, 29, S16–S18.
- Horvath, K. The Effect of GUNA-MDs in the Therapy Resistant Facial Paresis. In Proceedings of the International Congress of PRM, Low doses therapies, Prague, Czech Republic, 9 November 2013. Trials and Case Reports.
- Ding, L.; Yan, G.; Wang, B.; Xu, L.; Gu, Y.; Ru, T.; Cui, X.; Lei, L.; Liu, J.; Sheng, X.; et al. Transplantation of UC-MSCs on Collagen Scaffold Activates Follicles in Dormant Ovaries of POF Patients with Long History of Infertility. Sci. China Life Sci. 2018, 61, 1554–1565.
- Bechara, C.F.; Annambhotla, S.; Lin, P.H. Access Site Management with Vascular Closure Devices for Percutaneous Transarterial Procedures. J. Vasc. Surg. 2010, 52, 1682–1696.
- Awad, S.; Dharmavaram, S.; Wearn, C.S.; Dube, M.G.; Lobo, D.N. Effects of an Intraoperative Infusion of 4 Succinylated Gelatine (Gelofusine®) and 6 Hydroxyethyl Starch (Voluven®) on Blood Volume. Br. J. Anaesth. 2012, 109, 168–176.
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking Older Fibroblast Collapse and Therapeutic Implications. Arch. Dermatol. 2008, 144, 666–672.
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The Effect of Osteoarthritis Definition on Prevalence and Incidence Estimates: A Systematic Review. Osteoarthr. Cartil. 2011, 19, 1270–1285.
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42.
- Hartl, D.M.; Riquet, M.; Hans, S.; Laccourreye, O.; Vaissière, J.; Brasnu, D.F. Objective Voice Analysis after Autologous Fat Injection for Unilateral Vocal Fold Paralysis. Ann. Otol. Rhinol. Laryngol. 2001, 110, 229–235.
- Hirano, M.; Mori, K.; Tanaka, S.; Fujita, M. Vocal Function in Patients with Unilateral Vocal Fold Paralysis Before and After Silicone Injection. Acta. Otolaryngol. 1995, 115, 553–559.
- Swanson, N.A.; Stoner, J.G.; Siegle, R.J.; Solomon, A.R. Treatment Site Reactions to Zyderm Collagen Implantation. J. Dermatol. Surg. Oncol. 1983, 9, 377–380.
- Elson, M.L. The Role of Skin Testing in the Use of Collagen Injectable Materials. J. Dermatol. Surg. Oncol. 1989, 15, 301–303.
- Aragona, F.; D’Urso, L.; Marcolongo, R. Immunologic Aspects of Bovine Injectable Collagen in Humans. Eur. Urol. 1998, 33, 129–133.
- Zhou, Z.; Chen, X.; Zhou, X.; Yang, X.; Lu, D.; Kang, W.; Feng, X. Effects of Intraoperative Gelatin on Blood Viscosity and Oxygenation Balance. J. PeriAnesthesia Nurs. 2019, 34, 1274–1281.




