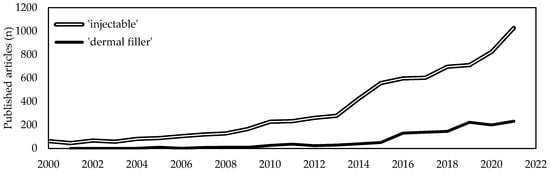
Soft tissues diseases significantly affect patients quality of life and usually require targeted, costly and sometimes constant interventions. With the average lifetime increase, a proportional increase of age-related soft tissues diseases has been witnessed. Due to this, the last two decades have seen a tremendous demand for minimally invasive one-step resolutive procedures. Intensive scientific and industrial research has led to the recognition of injectable formulations as a new advantageous approach in the management of complex diseases that are challenging to treat with conventional strategies. Among them, collagen-based products are revealed to be one of the most promising among bioactive biomaterials-based formulations. Collagen is the most abundant structural protein of vertebrate connective tissues and, because of its structural and non-structural role, is one of the most widely used multifunctional biomaterials in the health-related sectors, including medical care and cosmetics. Indeed, collagen-based formulations are historically considered as the “gold standard” and from 1981 have been paving the way for the development of a new generation of fillers. A huge number of collagen-based injectable products have been approved worldwide for clinical use and have routinely been introduced in many clinical settings for both aesthetic and regenerative surgery.
- collagen
- injectable collagen
- medical devices
1. Introduction
2. Collagen as Biomaterial
3. Collagen-Based Injectable Formulations
More than 60 kinds of collagen-based fillers are available on the market, according to the end-use and they have routinely been introduced in many clinical settings (Table 2). The most common collagen extraction sources for the manufacture of collagen based injectable formulations are bovine, swine, porcine, equine and human derived, whose advantages and disadvantages are described in depth elsewhere [19,20,25]. Bovine collagen is one of the most commonly used fillers for effectively reducing wrinkles and other facial imperfections. More famous branded bovine-based collagen fillers are Zyderm®, Zyplast®, Contigen® (Allergan Inc., Dublin, Ireland), Artefill® (Suneva Medical, San Diego, CA, USA), and Artecoll® (Canderm Pharma Inc., Saint-Laurent, QB, Canada). Others include CHondroGrid® (Bioteck Spa, Arcugnano, Italy), Integra Flowable Wound Matrix® (Integra LifeScience Corp., Princeton, NJ, USA), Resoplast® (Rofil Medical International, Breda, The Netherlands), Atelocell® (KOKEN Co., Ltd., Bunkyo-ku, Tokyo, Japan). However, bovine collagen is known to be exposed to zoonosis (e.g., the foot and mouth disease and the group of the bovine spongiform encephalopathies, among which the most dangerous for humans is the transmissible spongiform encephalopathy) and to trigger allergies (about 2–4% of population) [71,72,73]. In addition to the strict regulation to which all implantable products are subjected, two consecutive negative patient skin tests at 6 and 2 weeks are required before use [73,74]. This sensitivity has been considered generally acceptable for implants for human use and actually bovine collagen is principally used for the treatment of the integumental [6,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] (NCT01060943) and musculoskeletal apparatus [97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112] and to a minor extent for the gastrointestinal [113,114,115,116,117,118,119,120], urinary [65,121,122,123,124,125] and cardiovascular [126,127,128] systems. Recently, bovine collagen in fibrillar form has been employed as an organ protection system during thermal ablation of hepatic malignancies [129].
| Source | Manufacturer | Product | Additives | Applications | Ref. |
|---|---|---|---|---|---|
| Equine | Euroresearch S.r.l. (Milan, Italy) www.euroresearch.it, accessed on 14 February 2023 |
Nithya | – | Integumental | [163] |
| Linerase | – | Integumental | [164,165,166,167,179] | ||
| Nearmedic Italy S.r.l. (Como, Italy) www.salvecoll.com, accessed on 14 February 2023 |
Salvecoll-E | – | Integumental | [60] | |
| Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 |
Biocollagen gel | Type III collagen, bone spongy powder | Musculoskeletal | – | |
| Biocollagen crunch | Type III collagen, bone powder, bone spongy chips |
Musculoskeletal | – | ||
| ActivaBone CLX gel | Bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Injectable Paste |
Demineralized bone matrix, bone powder, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone modulable paste |
Demineralized bone matrix, bone powder, bone cortical and spongy granules, exur, Vitamin C | Musculoskeletal | – | ||
| ActivaBone Crunch | Demineralized bone matrix, bone powder, cortical and spongy chips, exur, Vitamin C | Musculoskeletal | – | ||
| Bovine | Bioteck Spa (Arcugnano, Italy) www.bioteck.com, accessed on 14 February 2023 |
CHondroGrid | – | Musculoskeletal | [112] |
| Integra LifeScience Corp. (Princeton, NJ, USA) www.integralife.com, accessed on 14 February 2023 |
Integra Flowable Wound Matrix | Glycosaminoglycans | Integumental | [88] | |
| Helitene | – | Soft tissues | [129] | ||
| Rofil Medical International (Breda, The Netherlands) | Resoplast | Lidocaine hydrochloride | Integumental | – | |
| Suneva Medical (San Diego, CA, USA) www.sunevamedical.com, accessed on 14 February 2023 |
ArteFill | Polymethylmethacrylate, lidocaine | Integumental | [75,77,78,79,80,81,82,83,84,85] | |
| Datascope Corp., (Montvale, NJ, USA) | VasoSeal | – | Cardiovascular | [128] | |
| BioMimetic Therapeutics, LLC (Franklin, TN, USA) www.biomimetics.com, accessed on 14 February 2023 |
Augment | β-tricalcium phosphate, recombinant human platelet-derived growth factor-BB | Musculoskeletal | [97,99,100,101,102,103,104,105,106,107,108,109,110,111] | |
| KOKEN Co., Ltd. (Bunkyo-ku, Tokyo, Japan) www.kokenmpc.co.jp, accessed on 14 February 2023 |
Atelocell | Type III collagen | Integumental, gastrointestinal |
[86,87,113,114], NCT01060943 | |
| B. Braun (Crissier, Switzerland) www.bbraun.com, accessed on 14 February 2023 |
Gelofusine | – | Cardiovascular | [126,127] | |
| Allergan, Inc. (Dublin, Ireland) www.abbvie.it, accessed on 14 February 2023 |
Zyplast | Glutaraldehyde | Integumental | [6,76,83,89,90,91,92,95,96,98,116,117,119,180] | |
| Zyderm | – | Integumental | [6,83,89,90,93,94,118,120,180] | ||
| Contigen | glutaraldehyde | Gastrointestinal and genitourinary | [115,121,122,123,124,125] | ||
| Swine | GUNA (Milan, Italy) www.guna.com, accessed on 14 February 2023 |
Dental Skin BioRegulation |
Vitamin C, magnesium gluconate, pyridozine chlorhydrate, riboflavin, thiamine chlorhydrate | Skin | [181] |
| Dental ATM BioRegulation |
Hypericum | Musculoskeletal | [130] | ||
| MD-HIP | Calcium phosphate | Musculoskeletal | [131] | ||
| MD-ISCHIAL | Rhododendron | Musculoskeletal | [132] | ||
| MD-KNEE | Arnica | Musculoskeletal | [133,143,144] | ||
| MD-LUMBAR | Hamemelis | Musculoskeletal | [132,134,135] | ||
| MD-NECK | Silicio | Musculoskeletal | – | ||
| MD-SHOULDERS | Iris | Musculoskeletal | [145,146] | ||
| MD-SMALL JOINTS | Viola | Musculoskeletal | – | ||
| MD-THORACIC | Cimifuga | Musculoskeletal | – | ||
| MD-MATRIX | Citric acid, nicotinamide | Soft tissues | [135,136,160] | ||
| MD-MUSCLE | Hypericum | Musculoskeletal | [130,132,133,134,135,136,137,146,160] | ||
| MD-POLY | Drosera | Musculoskeletal | – | ||
| MD-NEURAL | Citrullus | Musculoskeletal | [132,134,160] | ||
| MD-TISSUE | Ascorbic acid, magnesium gluconate, pyridoxine chlorhydrate, riboflavin, thiamine chlorhydrate | Soft tissues | – | ||
| Joint Biomaterials S.r.l. (Mestre, Italy) www.joint-biomateriali.it, accessed on 14 February 2023 |
CartiRegen | Fibrin glue | Musculoskeletal | – | |
| Ubiosis (Gyeonggi-do, Republic of Korea) www.ubiosis.com, accessed on 14 February 2023 |
COLTRIX CartiRegen | – | Musculoskeletal | – | |
| COLTRIX TendoRegen | – | Musculoskeletal | – | ||
| Sewon Cellontech Co., Ltd. (Seoul, Republic of Korea) www.swcell.com, accessed on 14 February 2023 |
CartiFill | Glucose, CaCl, amino acids, vitamin B, fibrin glue |
Musculoskeletal | [138,139], NCT02539030, NCT02519881 | |
| CartiZol | Glucose, CaCl, amino acids, vitamin B | Musculoskeletal | [140], NCT02539095 | ||
| RegenSeal | – | Musculoskeletal | [141] | ||
| TheraFill | – | Integumental | [86,87] | ||
| Sunmax Biotechnology Co., Ltd. (Tainan, Taiwan) www.sunmaxbiotech.com, accessed on 14 February 2023 |
Facial Gain | Lidocaine | Integumental | NCT03844529 | |
| Collagen Implant I | – | Integumental | – | ||
| Evolence (Skillman, NJ, USA) |
Dermicol-P35 | Ribose | Integumental | [2,147,148,149], NCT00929071, NCT00891774 | |
| Mentor Corp. (Santa Barbara, CA, USA) |
Fibrel | – | Integumental | [150,151] | |
| Tissue Science Labs. (Aldershot, UK) |
Permacol | – | Gastrointestinal | [153,154,155,156,157,158,159] | |
| EternoGen, LLC (Columbia, MO, USA) |
RPC Pure Collagen | Ethylenediamine tetraacetic acid | Integumental | [67] | |
| Aspid S.A. de C.V. (Mexico City, Mexico) www.aspidpharma.com, accessed on 14 February 2023 |
Fibroquel | Polyvinylpyrrolidone | Musculoskeletal | [161,162], NCT04517162 | |
| ColBar LifeScience Ltd. (Tel Aviv, Israel) www.ortho-dermatologics.com, accessed on 14 February 2023 |
Evolence | Ribose | Integumental | [147,152] | |
| Human | Fascia Biosystem (Beverly Hills, CA, USA) |
Fascian | Lidocain | Integumental | [6,168,171] |
| Fibrocell Science (Exton, PA, USA) www.fibrocell.com, accessed on 14 February 2023 |
Isolagen therapy | – | Integumental | NCT00655356 | |
| Inamed Corporation (Santa Barbara, CA, USA) www.inamed-cro.com, accessed on 14 February 2023 |
Cosmoplast | Glutaraldehyde, lidocaine hydrochloride | Integumental | [6,169] | |
| Cosmoderm | lidocaine hydrochloride | Integumental | [6,169] | ||
| Life Cell Corp. (Branchburg, NJ, USA) |
Dermalogen | Type and VI collagen, elastin, fibronectin, chondroitin sulfate, and other proteoglycans | Integumental | [170] | |
| Cymetra | Elascin, glycosaminoglycans, Lidocaine hydrochloride | Integumental | [6,118,172,173,174,175] | ||
| Collagenesis, Inc., (Beverly, MA, USA) |
Autologen | Elastin, fibronectin, glycosaminoglycans | Integumental | – | |
| Dermologen | - | Integumental | [173] | ||
| Plant | Vesco Pharmaceutical Co. Ltd. (Bangkok, Thailand) www.vescopharma.com, accessed on 14 February 2023 |
Collagen C 1000 | Vitamin C | Integumental | – |
| Silkworm | Monodermà (Milan, Italy) www.monoderma.com |
Fillagen | Hyaluronic acid, carboxymethylcellulose | Integumental | [178] |
| n. d. | Taumed (Rome, Italy) www.taumed.it, accessed on 14 February 2023 |
Karisma | Hyaluronic acid, carboxymethylcellulose | Integumental | – |
| n. d. | LABO International S.r.l. (Padova, Italy) www.labosuisse.com, accessed on 14 February 2023 |
Fillerina con 3D collagen |
Hyaluronic acid | Integumental | – |
| n. d. | Hebey Mepha Pharm Group Co., Ltd. (Shandong, Hebei, China) www.mephacn.com, accessed on 14 February 2023 |
Collagen Plus | – | Integumental | – |
| n. d. | Pierre Mulot Laboratories (Paris, France) |
Neutroskin | Vitamin C | Integumental | – |
| n. d. | Elements Pharmaceuticals (Shijiazhuang, Hebei, China) www.elementspharma.com, accessed on 14 February 2023 |
Ele-collagen | Vitamin C, Vitamin B6 | Integumental | – |
| n. d. | Globus Medical (Audubon, PA, USA) www.globusmedical.com, accessed on 14 February 2023 |
Kinex Bioactive gel | Bioglass, hyaluronic acid | Musculoskeletal | – |
4. Clinical Efficacy of Collagen-Based Injectable Implants
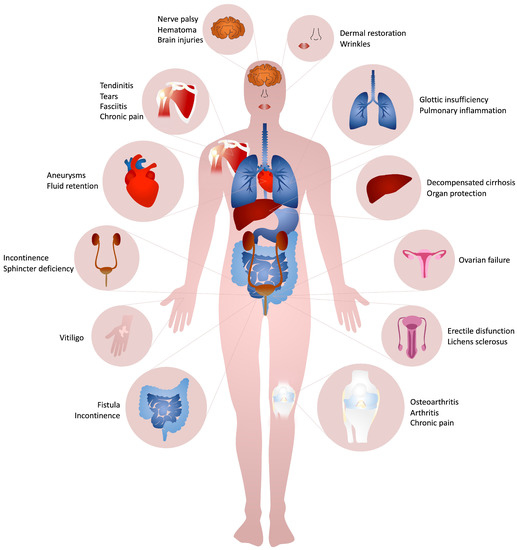
4.1. Integumental Apparatus
4.2. Musculoskeletal Apparatus
4.3. Urogenital System
4.4. Gastrointestinal Apparatus
5. Adverse Reactions to Collagen-Based Injectable Implants
This entry is adapted from the peer-reviewed paper 10.3390/polym15041020
