
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zachariah Grochau-Wright | -- | 2870 | 2023-05-03 16:47:21 | | | |
| 2 | Camila Xu | Meta information modification | 2870 | 2023-05-04 02:54:06 | | |
Video Upload Options
A group of green algae in the order of Volvocales provides an ideal model system for studying the transition from unicellular to differentiated multicellularity. This group—known as the volvocine algae—evolved multicellularity relatively recently (~240 million years ago) and contains extant relatives that span a range of complexities from unicellularity, to undifferentiated multicellularity, to differentiated multicellularity. The regA-like gene family within the volvocine algae serves as a model for the evolution of the genetic basis of cellular differentiation.
1. The Volvocine Model System
2. regA Gene Structure and Function
3. regA-like Gene Family Evolution
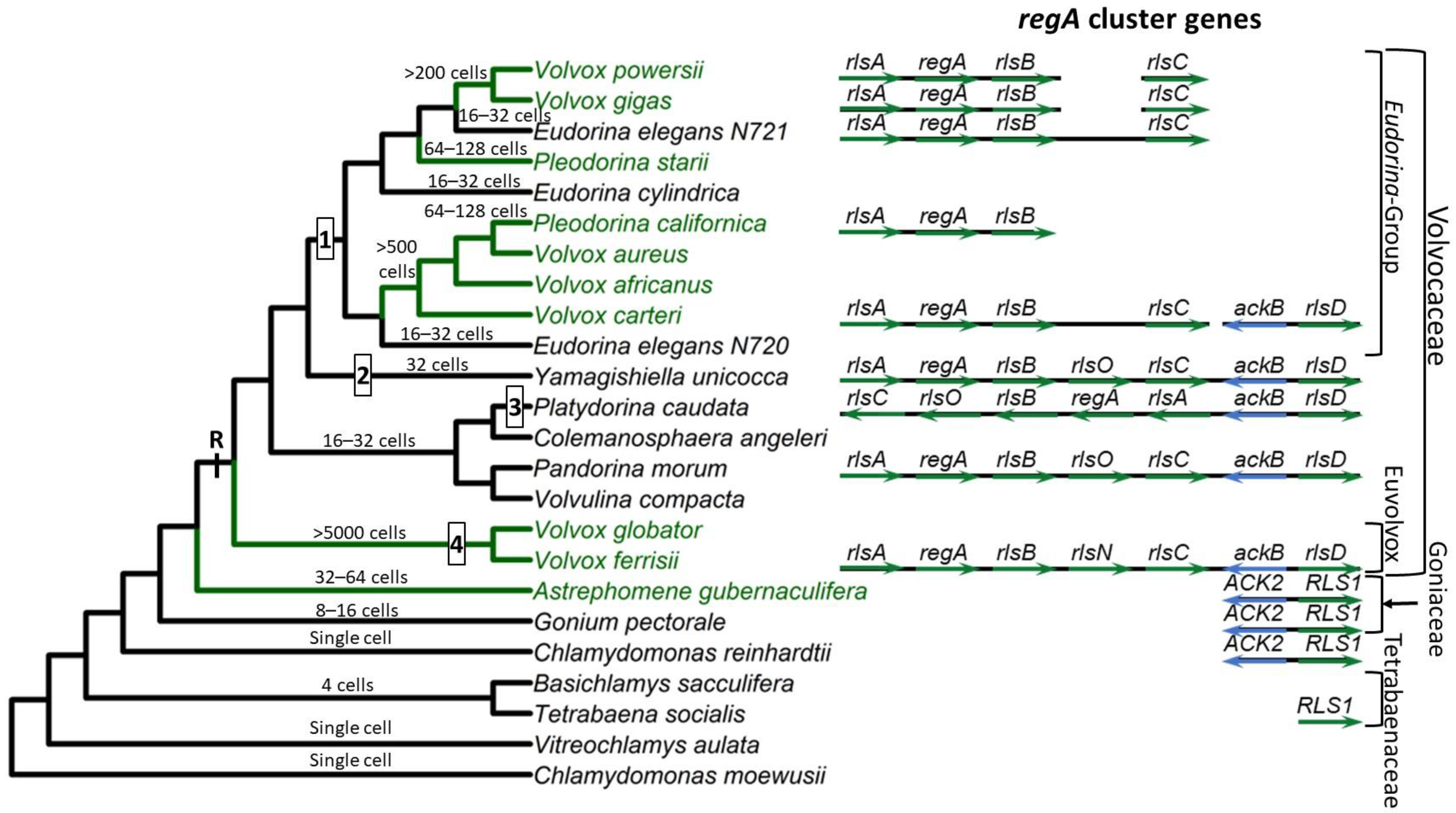
The VARL gene family is defined by the presence of a homologous VARL domain within the predicted protein (note that volvocine algae possess additional SAND-containing proteins outside the VARL family). Although all VARL genes contain the VARL domain, the sequence level conservation outside of the VARL domain is very low. Thus, entire gene sequences cannot be aligned and used for phylogenetic analyses. The VARL domain itself is very short (~86 amino acids) and not highly conserved, such that its utility for inferring evolutionary relationships between the members of the VARL gene family is also limited. Nevertheless, information from gene synteny, sequence signatures outside of the VARL domain, and the locations of conserved introns can help draw more robust conclusions regarding the evolution of the VARL family [25].
Based on currently available whole genome sequence data, the VARL gene family contains 12 members in C. reinhardtii [38], 8 in G. pectorale [8] and T. socialis [50], 6 in A. gubernaculifera [9], and 14 in V. carteri [38]. With the exception of regA orthologs (when present), all other regA homologs are known as regA-like sequences, annotated as RLS1-12 in Chlamydomonas and Goniaceae or rlsA-O in Volvocaceae. C. reinhardtii and other volvocine algae outside the Volvocaceae lack orthologs of any of the regA cluster genes. The closest homolog to the regA cluster genes found in these species is RLS1. This gene is an ortholog of the Volvocaceaen rlsD, which is the closest rls paralog of the regA cluster. Currently it is thought that the VARL gene family comprising several paralogs including RLS1/rlsD was already present in the common ancestor of all volvocine green algae. RLS1/rlsD underwent one or more duplication events in the common ancestor of the Volvocaceae family to give rise to a five-gene regA gene cluster comprising rlsA, regA, rlsB, rlsO, and rlsC. After the lineage leading to V. ferrisii diverged from the rest of the Volvocaceae, its rlsO gene gained a second VARL domain and evolved into rlsN. Meanwhile, the common ancestor of the Eudorina group lost rlsO. In addition, Y. unicocca lost two internal regA cluster genes (regA, rlsB, or rlsO) but restored the five-gene cluster via gene duplication, and the regA cluster of P. caudata became inverted relative to nearby syntenic markers (Figure 1).
Based on its role in suppressing reproduction in somatic cells, it has been hypothesized that regA evolved from a gene that was involved in trading off reproduction for survival (i.e., a life history trade-off gene) in the single-celled ancestors of V. carteri. Specifically, such a gene could have been co-opted by changing its expression from a temporal context (in response to an environmental cue) into a spatial context (in response to a developmental cue) [51].The common ancestor of V. carteri and C. reinhardtii likely had several VARL gene family members, one of which was RLS1. The RLS1 gene duplicated several times to give rise to the regA gene cluster in the common ancestor of the Volvocaceae, setting the stage for the functional co-option of regA during the evolution of cellular differentiation as well as other lineage-specific changes to regA cluster genes (Figure 1). The co-option of RLS1′s functions into a regA-like gene responsible for somatic cell differentiation likely involved the simulation of the ancestral environmentally induced signal in a developmental context.
References
- Coleman, A.W. A Comparative Analysis of the Volvocaceae (Chlorophyta). J. Phycol. 2012, 48, 491–513.
- Umen, J.G. Volvox and volvocine green algae. EvoDevo 2020, 11, 7–15.
- Herron, M.D.; Michod, R.E. Evolution of complexity in the volvocine algae: Transitions in individuality through Darwin’s eye. Evol. Int. J. Org. Evol. 2008, 62, 436–451.
- Kirk, D.L. A twelve-step program for evolving multicellularity and a division of labor. BioEssays News Rev. Mol. Cell. Dev. Biol. 2005, 27, 299–310.
- Nozaki, H.; Misawa, K.; Kajita, T.; Kato, M.; Nohara, S.; Watanabe, M.M. Origin and evolution of the colonial volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Mol. Phylogenet. Evol. 2000, 17, 256–268.
- Arakaki, Y.; Kawai-Toyooka, H.; Hamamura, Y.; Higashiyama, T.; Noga, A.; Hirono, M.; Olson, B.J.S.C.; Nozaki, H. The simplest integrated multicellular organism unveiled. PLoS ONE 2013, 8, e81641.
- Nozaki, H.; Itoh, M.; Watanabe, M.M.; Kuroiwa, T. Ultrastructure of the vegetative colonies and systematic position of basichlamys (Volvocales, Chlorophyta). Eur. J. Phycol. 1996, 31, 67–72.
- Hanschen, E.R.; Marriage, T.N.; Ferris, P.J.; Hamaji, T.; Toyoda, A.; Fujiyama, A.; Neme, R.; Noguchi, H.; Minakuchi, Y.; Suzuki, M.; et al. The Gonium pectorale genome demonstrates co-option of cell cycle regulation during the evolution of multicellularity. Nat. Commun. 2016, 7, 11370.
- Yamashita, S.; Yamamoto, K.; Matsuzaki, R.; Suzuki, S.; Yamaguchi, H.; Hirooka, S.; Minakuchi, Y.; Miyagishima, S.-Y.; Kawachi, M.; Toyoda, A.; et al. Genome sequencing of the multicellular alga Astrephomene provides insights into convergent evolution of germ-soma differentiation. Sci. Rep. 2021, 11, 22231.
- Nozaki, H.; Mahakham, W.; Athibai, S.; Yamamoto, K.; Takusagawa, M.; Misumi, O.; Herron, M.D.; Rosenzweig, F.; Kawachi, M. Rediscovery of the species of ‘ancestral Volvox’: Morphology and phylogenetic position of Pleodorina sphaerica (Volvocales, Chlorophyceae) from Thailand. ’ Phycologia 2017, 56, 469–475.
- Lindsey, C.R.; Rosenzweig, F.; Herron, M.D. Phylotranscriptomics points to multiple independent origins of multicellularity and cellular differentiation in the volvocine algae. BMC Biol. 2021, 19, 182.
- Grochau-Wright, Z.I.; Hanschen, E.R.; Ferris, P.J.; Hamaji, T.; Nozaki, H.; Olson, B.J.S.C.; Michod, R.E. Genetic Basis for Soma is Present in Undifferentiated Volvocine Green Algae. J. Evol. Biol. 2017, 30, 1205–1218.
- Herron, M.D.; Hackett, J.D.; Aylward, F.O.; Michod, R.E. Triassic origin and early radiation of multicellular volvocine algae. Proc. Natl. Acad. Sci. USA 2009, 106, 3254–3258.
- Kirk, D.L. Volvox: Molecular-Genetic Origins of Multicellularity and Cellular Differentiation; Cambridge University Press: Cambridge, UK, 1998.
- Kirk, D.L. Germ-soma differentiation in Volvox. Dev. Biol. 2001, 238, 213–223.
- Koufopanou, V. The Evolution of Soma in the Volvocales. Am. Nat. 1994, 143, 907–931.
- Kirk, M.M.; Ransick, A.; McRae, S.E.; Kirk, D.L. The Relationship between Cell Size and Cell Fate in Volvox carteri. J. Cell Biol. 1993, 123, 191–208.
- Grochau-Wright, Z.I. The Origin and Evolution of the Reg Cluster in the Volvocine Green Algae: A Model System for the Evolution of Cellular Differentiation. Ph.D. Dissertation, University of Arizona, Tucson, AZ, USA, 2019.
- Grochau-Wright, Z.I.; Ferris, P.J.; Tumberger, J.; Jiménez-Marin, B.; Olson, B.J.S.C.; Michod, R.E. Characterization and Transformation of reg Cluster Genes in Volvox powersii Enable Investigation of Convergent Evolution of Cellular Differentiation in Volvox. Protist 2021, 172, 125834.
- Ransick, A. Reproductive cell specification during Volvox obversus development. Dev. Biol. 1991, 143, 185–198.
- Ransick, A. Specification of reproductive cells in Volvox. In Evolutionary Conservation of Developmental Mechanisms, Proceedings of the 50th Symposium of the Society for Developmental Biology, Marquette University, Milwaukee, WI, USA, 20–23 June 1991; Spradling, A., Ed.; Wiley-Liss: Hoboken, NJ, USA, 1993; pp. 55–70.
- Sessoms, A.H.; Huskey, R.J. Genetic Control of Development in Volvox: Isolation and Characterization of Morphogenetic Mutants. Proc. Natl. Acad. Sci. USA 1973, 70, 1335–1338.
- Starr, R.C. Control of differentiation in Volvox. Dev. Biol. 1970, 4, 59–100.
- Huskey, R.J.; Griffin, B.E.; Cecil, P.O.; Callahan, A.M. A Preliminary Genetic Investigation of Volvox carteri. Genetics 1979, 91, 229–244.
- Huskey, R.J.; Griffin, B.E. Genetic Control of Somatic Cell Differentiation in Volvox. Dev. Biol. 1979, 72, 226–235.
- Baran, G. Analysis of Somatic Cell Differentiation in Volvox carteri f. nagariensis; University of Virginia: Charlottesville, VA, USA, 1984.
- Kirk, M.M.; Stark, K.; Miller, S.M.; Müller, W.; Taillon, B.E.; Gruber, H.; Schmitt, R.; Kirk, D.L. regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein. Development 1999, 126, 639–647.
- Harryman, A. Investigating the Roles of regA and Related Genes in the Evolution of Multicellularity in the Volvocine Green Algae. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2012.
- Klein, B.; Wibberg, D.; Hallmann, A. Whole transcriptome RNA-Seq analysis reveals extensive cell type-specific compartmentalization in Volvox carteri. BMC Biol. 2017, 15, 1–22.
- Matt, G.Y.; Umen, J.G. Cell-Type Transcriptomes of the Multicellular Green Alga Volvox carteri Yield Insights into the Evolutionary Origins of Germ and Somatic Differentiation Programs. G3 Genes|Genomes|Genet. 2018, 8, 531–550.
- König, S.G.; Nedelcu, A.M. The genetic basis for the evolution of soma: Mechanistic evidence for the co-option of a stress-induced gene into a developmental master regulator. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201414.
- Duncan, L.; Nishii, I.; Howard, A.; Kirk, D.; Miller, S.M. Orthologs and paralogs of regA, a master cell-type regulatory gene in Volvox carteri. Curr. Genet. 2006, 50, 61–72.
- Choi, G.; Przybylska, M.; Straus, D. Three abundant germ line-specific transcripts in Volvox carteri encode photosynthetic proteins. Curr. Genet. 1996, 30, 347–355.
- Meissner, M.; Stark, K.; Cresnar, B.; Kirk, D.L.; Schmitt, R. Volvox germline-specific genes that are putative targets of RegA repression encode chloroplast proteins. Curr. Genet. 1999, 36, 363–370.
- Tam, L.; Kirk, D.L. Identification of Cell-Type-Specific Characterization of their Expression Genes of Volvox carteri and during the Asexual Life Cycle. Dev. Biol. 1991, 145, 51–66.
- Stark, K.; Kirk, D.L.; Schmitt, R. Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carteri. Genes Dev. 2001, 15, 1449–1460.
- Babinger, K.; Hallmann, A.; Schmitt, R. Translational control of regA, a key gene controlling cell differentiation in Volvox carteri. Development 2006, 133, 4045–4051.
- Duncan, L.; Nishii, I.; Harryman, A.; Buckley, S.; Howard, A.; Friedman, N.R.; Miller, S.M. The VARL gene family and the evolutionary origins of the master cell-type regulatory gene, regA, in Volvox carteri. J. Mol. Evol. 2007, 65, 1–11.
- Bottomley, M.J.J.; Collard, M.W.W.; Huggenvik, J.I.I.; Liu, Z.; Gibson, T.J.J.; Sattler, M. The SAND domain structure defines a novel DNA-binding fold in transcriptional regulation. Nat. Struct. Biol. 2001, 8, 626–633.
- Barker, H.E.; Smyth, G.K.; Wettenhall, J.; Ward, T.A.; Bath, M.L.; Lindeman, G.J.; Visvader, J.E. Deaf-1 regulates epithelial cell proliferation and side-branching in the mammary gland. BMC Dev. Biol. 2008, 8, 94.
- Veraksa, A.; Kennison, J.; McGinnis, W. DEAF-1 function is essential for the early embryonic development of Drosophila. Genesis 2002, 33, 67–76.
- Nakagawa, T.; Tsuruma, K.; Uehara, T.; Nomura, Y. GMEB1, a novel endogenous caspase inhibitor, prevents hypoxia- and oxidative stress-induced neuronal apoptosis. Neurosci. Lett. 2008, 438, 34–37.
- Kulkarni, M.; Shakes, D.C.; Guevel, K.; Smith, H.E. SPE-44 Implements Sperm Cell Fate. PLoS Genet. 2012, 8, e1002678.
- Radhakrishnan, K.; Bhagya, K.P.; Kumar, A.T.; Devi, A.N.; Sengottaiyan, J.; Kumar, P.G. Autoimmune Regulator (AIRE) Is Expressed in Spermatogenic Cells, and It Altered the Expression of Several Nucleic-Acid-Binding and Cytoskeletal Proteins in Germ Cell 1 Spermatogonial (GC1-spg) Cells. Mol. Cell. Proteom. 2016, 15, 2686–2698.
- Schaller, C.E.; Wang, C.L.; Beck-engeser, G.; Goss, L.; Scott, H.S.; Anderson, M.S.; Wabl, M. Expression of Aire and the Early Wave of Apoptosis in Spermatogenesis. J. Immunol. 2008, 180, 1338–1343.
- Chen, L.-Q.; Luo, J.-H.; Cui, Z.-H.; Xue, M.; Wang, L.; Zhang, X.-Y.; Pawlowski, W.P.; He, Y. ATX3, ATX4, and ATX5 Encode Putative H3K4 Methyltransferases and Are Critical for Plant Development. Plant Physiol. 2017, 174, 1795–1806.
- Carles, C.C. ULTRAPETALA1 encodes a SAND domain putative transcriptional regulator that controls shoot and floral meristem activity in Arabidopsis. Development 2005, 132, 897–911.
- Carles, C.C.; Fletcher, J.C. The SAND domain protein ULTRAPETALA1 acts as a trithorax group factor to regulate cell fate in plants. Genes Dev. 2009, 23, 2723–2728.
- Fletcher, J.C. The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 2001, 128, 1323–1333.
- Featherston, J.; Arakaki, Y.; Hanschen, E.R.; Ferris, P.J.; Michod, R.E.; Olson, B.J.S.C.; Nozaki, H.; Durand, P.M. The 4-celled tetrabaena socialis nuclear genome reveals the essential components for genetic control of cell number at the origin of multicellularity in the volvocine lineage. Mol. Biol. Evol. 2018, 35, 855–870.
- Nedelcu, A.M.; Michod, R.E. The evolutionary origin of an altruistic gene. Mol. Biol. Evol. 2006, 23, 1460–1464.




