
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hideo Yamasaki | -- | 1471 | 2023-04-28 04:43:39 | | | |
| 2 | Catherine Yang | Meta information modification | 1471 | 2023-04-28 05:42:24 | | |
Video Upload Options
Nitric oxide (NO) is a gaseous free radical that is largely produced by the enzyme NO synthase (NOS) in cells. NO produced by upper epidermal cells contributes to the inactivation of viruses and bacteria contained in air or aerosols. In addition to enzymatic production, NO can be generated by the chemical reduction of inorganic nitrite (NO2−), an alternative mechanism for NO production in living organisms. Dietary vitamin C, largely contained in fruits and vegetables, can reduce the nitrite in saliva to produce NO in the oral cavity when chewing foods. In the stomach, salivary nitrite can also be reduced to NO by vitamin C secreted from the epidermal cells of the stomach. The strong acidic pH of gastric juice facilitates the chemical reduction of salivary nitrite to produce NO. It is evident that NO exhibits substantial antiviral activity against many types of viruses, including SARS-CoV-2.
1. Smokers’ Paradox
2. RNS Biochemistry
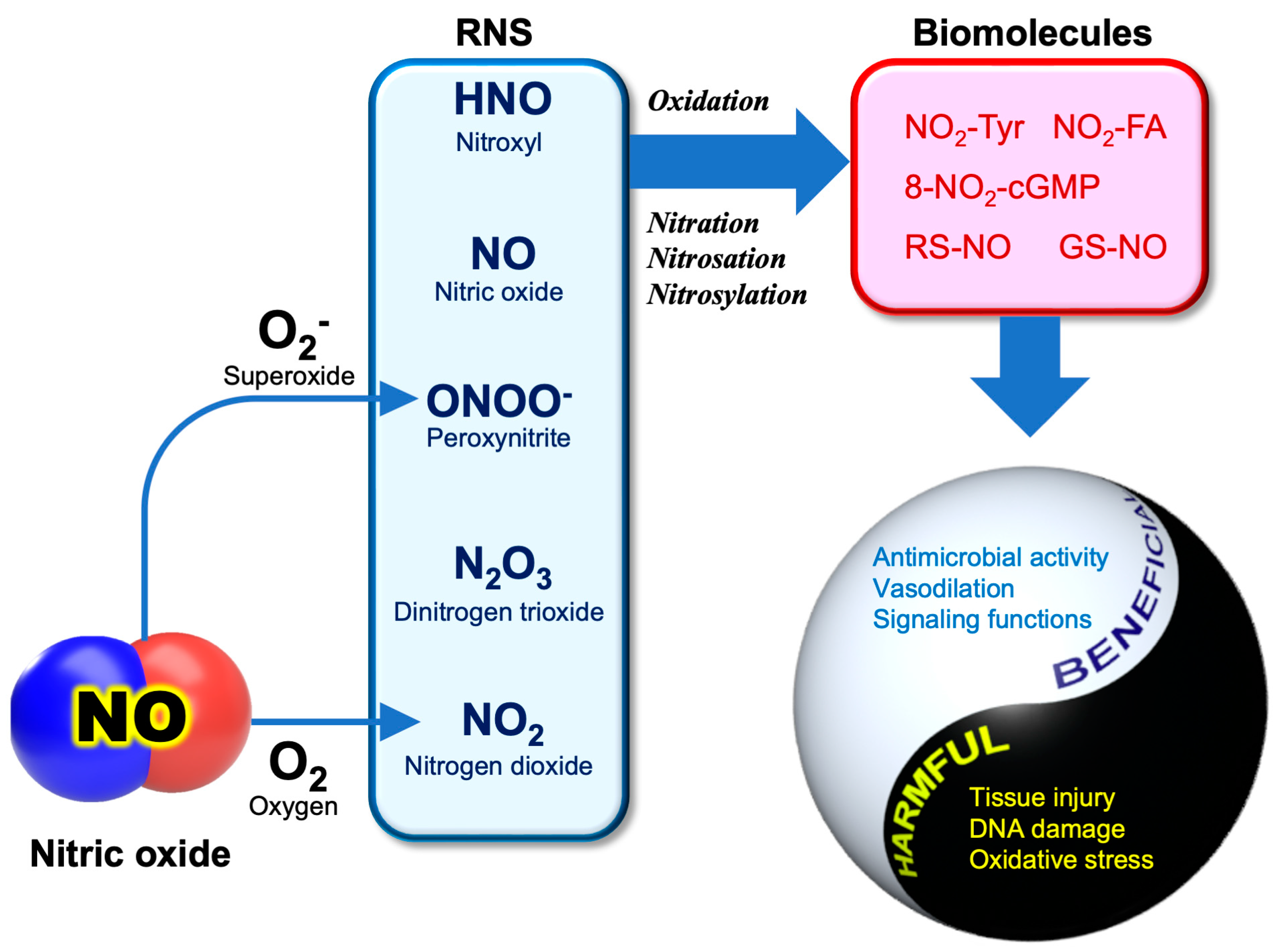
3. Anti-SARS-CoV-2 Activity of NO
References
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741.
- Lombardi, C.; Gani, F.; Berti, A.; Comberiati, P.; Peroni, D.; Cottini, M. Asthma and COVID-19: A dangerous liaison? Asthma Res. Pract. 2021, 7, 9.
- Sunjaya, A.P.; Allida, S.M.; Di Tanna, G.L.; Jenkins, C. Asthma and risk of infection, hospitalization, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J. Asthma 2022, 59, 866–879.
- Morais-Almeida, M.; Aguiar, R.; Martin, B.; Ansotegui, I.J.; Ebisawa, M.; Arruda, L.K.; Caminati, M.; Canonica, G.W.; Carr, T.; Chupp, G.; et al. COVID-19, asthma, and biologic therapies: What we need to know. World Allergy Organ. J. 2020, 13, 100126.
- Sandrini, A.; Taylor, D.R.; Thomas, P.S.; Yates, D.H. Fractional exhaled nitric oxide in asthma: An update. Respirology 2010, 15, 57–70.
- Farsalinos, K.; Barbouni, A.; Poulas, K.; Polosa, R.; Caponnetto, P.; Niaura, R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: A systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2020, 11, 2040622320935765.
- Miyara, M.; Tubach, F.; Pourcher, V.; Morelot-Panzini, C.; Pernet, J.; Haroche, J.; Lebbah, S.; Morawiec, E.; Gorochov, G.; Caumes, E.; et al. Low rate of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios 2020, WPP19W.4.
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Patel, U.K.; Shahid, I.; Ahmed, J.; Kalra, A.; Michos, E.D. Is there a smoker’s paradox in COVID-19? BMJ Evidence-Based Med. 2021, 26, 279–284.
- Hedenstierna, G.; Chen, L.; Hedenstierna, M.; Lieberman, R.; Fine, D.H. Nitric oxide dosed in short bursts at high concentrations may protect against COVID 19. Nitric Oxide 2020, 103, 1–3.
- Akaike, T.; Maeda, H. Nitric oxide and virus infection. Immunology 2000, 101, 300–308.
- DeGroote, M.A.; Fang, F.C. Antimicrobial properties of nitric oxide. In Nitric Oxide and Infection; Fang, F.C., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 231–261.
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The reactive rpecies Iinteractome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 2017, 27, 684–712.
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Biophys. Acta 1999, 1411, 385–400.
- Nag, T.C.; Kathpalia, P.; Gorla, S.; Wadhwa, S. Localization of nitro-tyrosine immunoreactivity in human retina. Ann. Anat. 2019, 223, 8–18.
- Akaike, T.; Okamoto, S.; Sawa, T.; Yoshitake, J.; Tamura, F.; Ichimori, K.; Miyazaki, K.; Sasamoto, K.; Maeda, H. 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 685–690.
- Villacorta, L.; Gao, Z.; Schopfer, F.J.; Freeman, B.A.; Chen, Y.E. Nitro-fatty acids in cardiovascular regulation and diseases: Characteristics and molecular mechanisms. Front. Biosci. 2016, 21, 873.
- Sakihama, Y.; Tamaki, R.; Shimoji, H.; Ichiba, T.; Fukushi, Y.; Tahara, S.; Yamasaki, H. Enzymatic nitration of phytophenolics: Evidence for peroxynitrite-independent nitration of plant secondary metabolites. FEBS Lett. 2003, 553, 377–380.
- Fukuto, J.M.; Perez-Ternero, C.; Zarenkiewicz, J.; Lin, J.; Hobbs, A.J.; Toscano, J.P. Hydropersulfides (RSSH) and nitric oxide (NO) signaling: Possible effects on S-nitrosothiols (RS-NO). Antioxidants 2022, 11, 169.
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004, 423, 12–22.
- Yamasaki, H. Nitrite-dependent nitric oxide production pathway: Implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1477–1488.
- Toda, N.; Ayajiki, K.; Okamura, T. Control of systemic and pulmonary blood pressure by nitric oxide formed through neuronal nitric oxide synthase. J. Hypertens. 2009, 27, 1929–1940.
- Szabo, C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 2003, 140-141, 105–112.
- Sanchez, A.G.; Ibargoyen, M.N.; Mastrogiovanni, M.; Radi, R.; Keszenman, D.J.; Peluffo, R.D. Fast and biphasic 8-nitroguanine production from guanine and peroxynitrite. Free Radic. Biol. Med. 2022, 193, 474–484.
- Akaike, T.; Fujii, S.; Kato, A.; Yoshitake, J.; Miyamoto, Y.; Sawa, T.; Okamoto, S.; Suga, M.; Asakawa, M.; Nagai, Y.; et al. Viral mutation accelerated by nitric oxide production during infectionin vivo. FASEB J. 2000, 14, 1447–1454.
- Oza, P.P.; Kashfi, K. Utility of NO and H2S donating platforms in managing COVID-19: Rationale and promise. Nitric Oxide 2022, 128, 72–102.
- Klingstrom, J.; Akerstrom, S.; Hardestam, J.; Stoltz, M.; Simon, M.; Falk, K.I.; Mirazimi, A.; Rottenberg, M.; Lundkvist, A. Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. Eur. J. Immunol. 2006, 36, 2649–2657.
- Saura, M.; Zaragoza, C.; McMillan, A.; Quick, R.A.; Hohenadl, C.; Lowenstein, J.M.; Lowenstein, C.J. An antiviral mechanism of nitric oxide: Inhibition of a viral protease. Immunity 1999, 10, 21–28.
- Colasanti, M.; Persichini, T.; Venturini, G.; Ascenzi, P. S-nitrosylation of viral proteins: Molecular bases for antiviral effect of nitric oxide. IUBMB Life 1999, 48, 25–31.
- Akerstrom, S.; Mousavi-Jazi, M.; Klingstrom, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1966–1969.
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226.
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Jarhult, J.D.; Lennerstrand, J.; Lundkvist, A. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020, 37, 101734.
- Guns, J.; Vanherle, S.; Hendriks, J.J.A.; Bogie, J.F.J. Protein lipidation by palmitate controls macrophage function. Cells 2022, 11, 565.
- Akerstrom, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9.
- Wong, N.A.; Saier, M.H., Jr. The SARS-coronavirus infection cycle: A survey of viral membraneproteins, their functional interactions and pathogenesis. Int. J. Mol. Sci. 2021, 22, 1308.
- Li, D.; Liu, Y.; Lu, Y.; Gao, S.; Zhang, L. Palmitoylation of SARS-CoV-2 S protein is critical for S-mediated syncytia formation and virus entry. J. Med. Virol. 2022, 94, 342–348.
- Main, A.; Fuller, W. Protein S-palmitoylation: Advances and challenges in studying a therapeutically important lipid modification. FEBS J. 2022, 289, 861–882.
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468.
- Fernandez-Hernando, C.; Fukata, M.; Bernatchez, P.N.; Fukata, Y.; Lin, M.I.; Bredt, D.S.; Sessa, W.C. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J. Cell Biol. 2006, 174, 369–377.
- Li, X.; Yuan, H.; Li, X.; Wang, H. Spike protein mediated membrane fusion during SARS-CoV-2 infection. J. Med. Virol. 2022, 95, e28212.




