
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wenxu Zhang | -- | 788 | 2023-04-24 03:36:45 | | | |
| 2 | Jessie Wu | + 1062 word(s) | 1850 | 2023-04-24 03:44:28 | | | | |
| 3 | Jessie Wu | + 109 word(s) | 1959 | 2023-04-24 03:45:11 | | | | |
| 4 | Wenxu Zhang | + 8 word(s) | 1967 | 2023-04-24 04:57:44 | | | | |
| 5 | Jessie Wu | + 5 word(s) | 1972 | 2023-04-24 05:27:38 | | |
Video Upload Options
The pollution and scarcity of freshwater resources are global problems that have a significant influence on human life. It is very important to remove harmful substances in the water to realize the recycling of water resources. Hydrogels have recently attracted attention due to their special three-dimensional network structure, large surface area, and pores, which show great potential for the removal of pollutants in water. In their preparation, natural polymers are one of the preferred materials because of their wide availability, low cost, and easy thermal degradation. However, when it is directly used for adsorption, its performance is unsatisfactory, so it usually needs to be modified in the preparation process.
1. Adsorption Mechanism and Kinetics of Polysaccharide-Based Hydrogels
1.1. Adsorption Mechanism
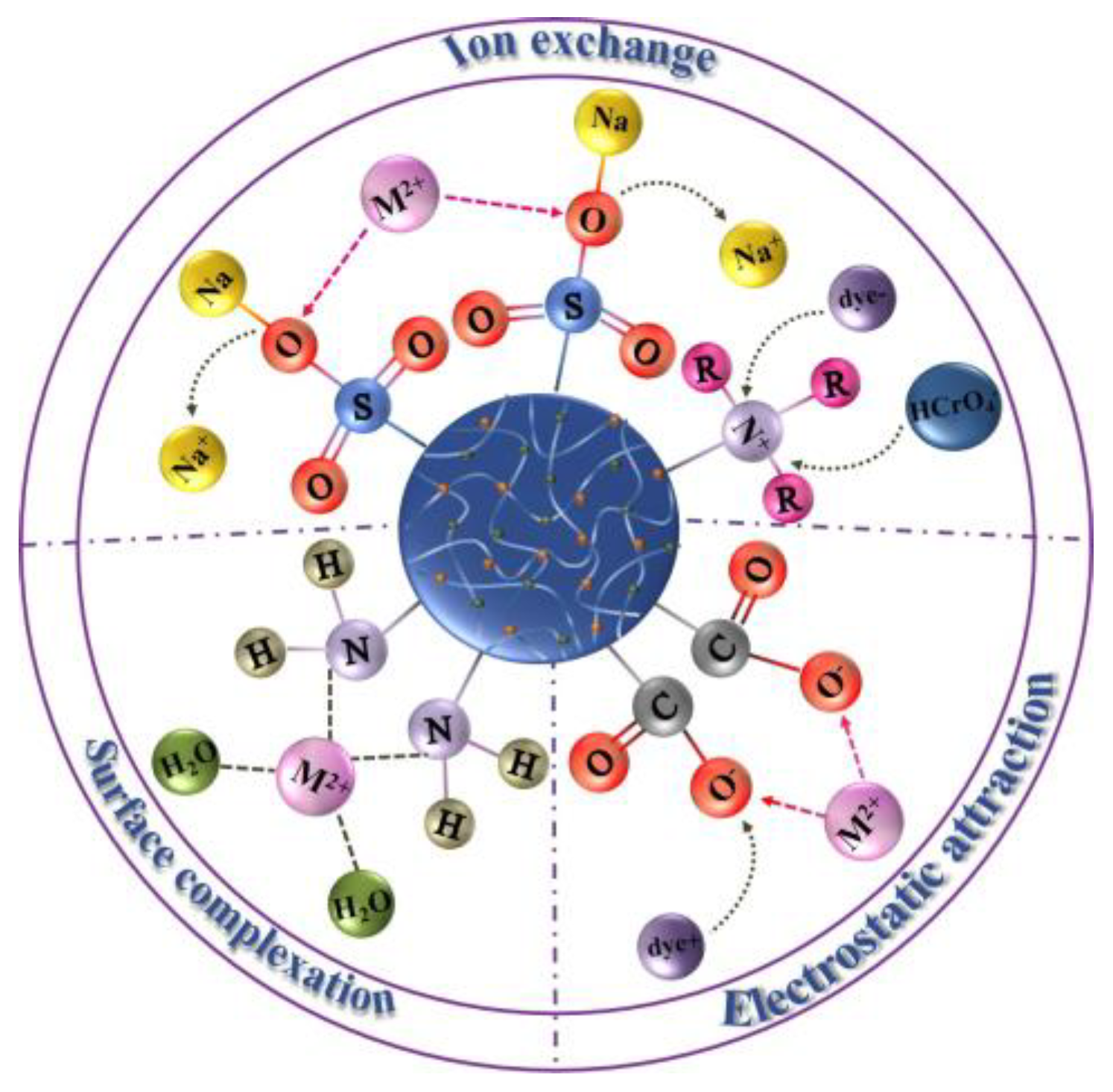
1.2. Adsorption Kinetics
1.2.1. Pseudo-First-Order Kinetic
1.2.2. Pseudo-Second-Order Kinetic
1.3. Adsorption Isotherms
1.3.1. Langmuir Isotherm Equation
1.3.2. Freundlich Isotherm Equation
2. Adsorption Applications of Polysaccharide-Based Hydrogels
2.1. Heavy Metal Ion Adsorption
| Polysaccharide Hydrogel Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. |
|---|---|---|---|---|---|
| AM/AA | Cu2+ | 157.51 | Langmuir | PSO | [13] |
| Pd2+ | 393.28 | Langmuir | PSO | ||
| Cd2+ | 289.97 | Langmuir | PSO | ||
| Nanocellulose/Carbon dot | Cr6+ | 599.9 | Freundlich | PSO | [14] |
| Straw cellulose | Cd2+ | 95.62 | Langmuir | PSO | [15] |
| Nanocellulose/SA | Pb2+ | 318.47 | Langmuir | PSO | [16] |
| GO-PVA-CS | Cd2+ | 172.11 | Langmuir | PSO | [17] |
| Ni2+ | 70.37 | Langmuir | PSO | ||
| CYCS/CNC | Pb2+ | 334.92 | Langmuir | PSO | [18] |
| Chitosan oligosaccharide | Cr6+ | 148.1 | Langmuir | PSO | [19] |
| α-ketoglutaric acid–PAM-CS | Cu2+ | 72.39 | Langmuir | PSO | [20] |
| Pd2+ | 61.41 | Langmuir | PSO | ||
| Zn2+ | 51.89 | Langmuir | PSO | ||
| CPCS-PAM-PVA | Cr6+ | 95.31 | Langmuir | PFO | [21] |
| Millettia speciosa Champ cellulose-CS | Cu2+ | 23.37 | Freundlich | PFO | [22] |
| All-lignocellulose | Cu2+ | 350 | Langmuir | PSO | [23] |
| Caffeic acid starch | Cr6+ | 96.45 | Langmuir | PSO | [24] |
| Starch-FMBO | As3+ | 161.29 | Langmuir | PSO | [25] |
| Starch nanoparticle | Pb2+ | 40.52 | Langmuir | PSO | [26] |
| Cu2+ | 32.88 | Langmuir | PSO | ||
| dibenzo-18-crown-6 starch | Cd2+ | 368.5 | Freundlich | PSO | [27] |
| Ni2+ | 182.5 | Freundlich | PSO | ||
| Zn2+ | 377.5 | Freundlich | PSO | ||
| Cu2+ | 385 | Freundlich | PSO | ||
| Succinic anhydride-SNCs | Cu2+ | 84.07 | Freundlich | PSO | [28] |
| PVA-SA | Pb2+ | 784.97 | Langmuir | PSO | [29] |
| ZIF-67-SA | Cu2+ | 153.63 | Langmuir | PSO | [30] |
| AM-GO-SA | Cu2+ | 68.76 | Langmuir | PSO | [31] |
| Pb2+ | 240.69 | Langmuir | PSO | ||
| Zeolite-PVA-SA | Pb2+ | 99.5 | Langmuir | PFO | [32] |
| Cd2+ | 99.2 | Langmuir | PFO | ||
| Sr2+ | 98.8 | Langmuir | PFO | ||
| Cu2+ | 97.2 | Langmuir | PFO | ||
| Zn2+ | 95.6 | Langmuir | PFO | ||
| Ni2+ | 93.1 | Langmuir | PFO | ||
| Mn2+ | 92.4 | Langmuir | PFO | ||
| Starch ether-SA | Cu2+ | 25.81 | Langmuir | PSO | [33] |
| Reptilite-Starch | Pb2+ | 180.8 | Langmuir | PSO | [34] |
| NCDs-CNF/CS | Cu2+ | 148.3 | Langmuir | PSO | [35] |
| Cr6+ | 294.46 | Langmuir | PSO | ||
| CTS/CA/BT | Pb2+ | 434.89 | Freundlich | PSO | [36] |
| Cu2+ | 115.30 | Freundlich | PSO | ||
| Cd2+ | 102.38 | Freundlich | PSO | ||
| GO-SA | As5+ | 277.39 | Langmuir | PSO | [37] |
| PAN-PPY-SA-GO | Cu2+ | 133.7 | Redlich–Peterson | PFO | [38] |
| Cr6+ | 87.2 | Redlich–Peterson | PFO |
2.2. Dye Adsorption
| Polysaccharide Hydrogels Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. |
|---|---|---|---|---|---|
| C/SA/Fe | MB | 105.93 | Langmuir | PSO | [43] |
| Carboxymethylcellulose | MB | 756 | Freundlich | PSO | [44] |
| Pineapple peel cellulose/diatomite |
MB | 101.94 | Langmuir | PSO | [45] |
| PCMC-PVA | MB | 172.14 | Langmuir | PSO | [46] |
| All-lignocellulose | MB | 145 | Langmuir | PSO | [23] |
| Millettia speciosa Champ cellulose-CS | CR | 221.43 | Freundlich | PSO | [22] |
| PAM-Fe3O4-CS | MB | 1603 | Langmuir | PFO | [47] |
| Montmorillonite-CS | MB | 530 | Langmuir | PSO | [48] |
| GO-CS-Fe3O4 | MB | 289 | Langmuir | PSO | [49] |
| EBT | 292 | Langmuir | PSO | [50] | |
| Jute cellulose nanocrystal | MB | 131.58 | Langmuir | PSO | |
| Succinic anhydride-SNCs | MB | 84.00 | Freundlich | PSO | [28] |
| Reptilite-Starch | MB | 277.0 | Langmuir | PSO | [34] |
| PAM-cassava starch | MB | 2000 | Langmuir | PSO | [51] |
| MXene-SA | MB | 92.17 | Langmuir | PSO | [52] |
| AA-GO-SA | MG | 628.93 | Langmuir | PSO | [53] |
| Flax seed ash-SA | MB | 333.3 | Langmuir | PSO | [54] |
| PEI-SA | MB | 400 | Langmuir | PSO | [55] |
| AM-HEMA-Starch | MG | 164 | Langmuir | PFO | [22] |
| MV | 156 | Freundlich | PFO |
2.3. Drug Antibiotics Adsorption
| Polysaccharide Hydrogels Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. |
|---|---|---|---|---|---|
| Fe3O4-Starch | Naphthalene | 24.752 | Langmuir | PSO | [56] |
| CS-Chitosan film | Cefotaxime Sodium | 1003.64 | Freundlich | PSO | [57] |
| GO-SA | Tetracycline | 477.9 | Freundlich | PSO | [37] |
| Amino/GO-SA | Ciprofloxacin | 301.36 | Langmuir | PSO | [58] |
| Humicacid-CS-Biochar | Ciprofloxacin | 154.89 | Langmuir | PSO | [59] |
| Biochar-CS | Ciprofloxacin | 106.038 | Langmuir | PSO | [60] |
| Enrofloxacin | 100.433 | Langmuir | PSO | ||
| GO-SA | Fluoxacin | 4.11 | Langmuir | PSO | [61] |
| Moxifloxacin | 3.43 | Langmuir | PSO | ||
| Fe3O4-SA | Tetracycline | 454.54 | Langmuir | PSO | [62] |
| Amoxicillin | 400 | Langmuir | PSO | ||
| Trimethylammonium chloride-CS | Tetracycline | 22.42 | Langmuir | PFO | [63] |
| PVA-SA-Cu2+ | Tetracycline | 231.431 | Langmuir | PSO | [64] |
References
- Can, V.; Kochovski, Z.; Reiter, V.; Severin, N.; Siebenbürger, M.; Kent, B.; Just, J.; Rabe, J.P.; Ballauff, M.; Okay, O. Nanostructural Evolution and Self-Healing Mechanism of Micellar Hydrogels. Macromolecules 2016, 49, 2281–2287.
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463.
- Largitte, L.; Pasquier, R. New models for kinetics and equilibrium homogeneous adsorption. Chem. Eng. Res. Des. 2016, 112, 289–297.
- Rodríguez-Narciso, S.; Lozano-Álvarez, J.A.; Salinas-Gutiérrez, R.; Castañeda-Leyva, N.; Bonilla-Petriciolet, A. A Stochastic Model for Adsorption Kinetics. Adsorpt. Sci. Technol. 2021, 2021, 1–21.
- Arroyave, J.M.; Avena, M.; Tan, W.; Wang, M. The two-species phosphate adsorption kinetics on goethite. Chemosphere 2022, 307, 135782.
- Cui, Z.; Wen, J.; Chen, J.; Xue, Y.; Feng, Y.; Duan, H.; Ji, B.; Li, R. Diameter dependent thermodynamics of adsorption on nanowires: A theoretical and experimental study. Chem. Eng. Sci. 2022, 247, 117061.
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. B Biointerfaces 2020, 191, 110992.
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279.
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959.
- Wang, X.; Wang, Y.; He, S.; Hou, H.; Hao, C. Ultrasonic-assisted synthesis of superabsorbent hydrogels based on sodium lignosulfonate and their adsorption properties for Ni(2). Ultrason. Sonochem. 2018, 40, 221–229.
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019, 169, 45–54.
- Wu, D.; Gao, Y.; Li, W.; Zheng, X.; Chen, Y.; Wang, Q. Selective Adsorption of La3+ Using a Tough Alginate-Clay-Poly(n-isopropylacrylamide) Hydrogel with Hierarchical Pores and Reversible Re-Deswelling/Swelling Cycles. ACS Sustain. Chem. Eng. 2016, 4, 6732–6743.
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022.
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr(VI). Sci Total Environ. 2021, 760, 143395.
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445.
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122.
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd(2+) and Ni(2+) from Aqueous Single-Metal Solutions on Graphene Oxide-Chitosan-Poly(vinyl alcohol) Hydrogels. Langmuir 2019, 35, 4481–4490.
- Li, Y.; Yang, Y.; Huang, Z.; Luo, Z.; Qian, C.; Li, Y.; Duan, Y. Preparation of low molecular chitosan by microwave-induced plasma desorption/ionization technology. Int. J. Biol. Macromol. 2021, 187, 441–450.
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A novel tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel for total adsorption of Cr(VI). Carbohydr. Polym. 2019, 224, 115154.
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262.
- Cao, J.; He, G.; Ning, X.; Wang, C.; Fan, L.; Yin, Y.; Cai, W. Hydroxypropyl chitosan-based dual self-healing hydrogel for adsorption of chromium ions. Int. J. Biol. Macromol. 2021, 174, 89–100.
- Chen, X.; Huang, Z.; Luo, S.-Y.; Zong, M.-H.; Lou, W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198.
- Melilli, G.; Yao, J.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Photocurable “all-lignocellulose” derived hydrogel nanocomposites for adsorption of cationic contaminants. Sustain. Mater. Technol. 2021, 27, e00243.
- Liu, F.; Hua, S.; Wang, C.; Qiu, M.; Jin, L.; Hu, B. Adsorption and reduction of Cr(VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 2021, 279, 130539.
- Xu, F.; Chen, H.; Dai, Y.; Wu, S.; Tang, X. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J. Environ. Manag. 2019, 245, 160–167.
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent trends in the application of modified starch in the adsorption of heavy metals from water: A review. Carbohydr. Polym. 2021, 269, 117763.
- Ibrahim, B.M.; Fakhre, N.A. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80.
- Chen, Q.J.; Zheng, X.M.; Zhou, L.L.; Zhang, Y.F. Adsorption of Cu(II) and Methylene Blue by Succinylated Starch Nanocrystals. Starch-Stärke 2019, 71, 1800266.
- Wang, W.; Liu, X.; Wang, X.; Zong, L.; Kang, Y.; Wang, A. Fast and Highly Efficient Adsorption Removal of Toxic Pb(II) by a Reusable Porous Semi-IPN Hydrogel Based on Alginate and Poly(Vinyl Alcohol). Front. Chem. 2021, 9, 662482.
- Li, Z.; Guo, Z.; Zhang, T.; Li, Q.; Chen, J.; Ji, W.; Liu, C.; Wei, Y. Fabrication of in situ ZIF-67 grown on alginate hydrogels and its application for enhancing Cu (II) adsorption from aqueous solutions. Colloids Surf. B Biointerfaces 2021, 207, 112036.
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653.
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716.
- Dai, M.; Liu, Y.; Ju, B.; Tian, Y. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(II) from aqueous solution. Bioresour. Technol. 2019, 294, 122192.
- Wang, F.; Chang, P.R.; Zheng, P.; Ma, X. Monolithic porous rectorite/starch composites: Fabrication, modification and adsorption. Appl. Surf. Sci. 2015, 349, 251–258.
- Chen, X.; Song, Z.; Yuan, B.; Li, X.; Li, S.; Thang Nguyen, T.; Guo, M.; Guo, Z. Fluorescent carbon dots crosslinked cellulose Nanofibril/Chitosan interpenetrating hydrogel system for sensitive detection and efficient adsorption of Cu (II) and Cr (VI). Chem. Eng. J. 2022, 430, 133154.
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891.
- He, J.; Ni, F.; Cui, A.; Chen, X.; Deng, S.; Shen, F.; Huang, C.; Yang, G.; Song, C.; Zhang, J.; et al. New insight into adsorption and co-adsorption of arsenic and tetracycline using a Y-immobilized graphene oxide-alginate hydrogel: Adsorption behaviours and mechanisms. Sci. Total Environ. 2020, 701, 134363.
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y.; et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358.
- Veregue, F.R.; de Lima, H.H.C.; Ribeiro, S.C.; Almeida, M.S.; da Silva, C.T.P.; Guilherme, M.R.; Rinaldi, A.W. MCM-41/chondroitin sulfate hybrid hydrogels with remarkable mechanical properties and superabsorption of methylene blue. Carbohydr. Polym. 2020, 247, 116558.
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and beta-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372.
- Beyranvand, N.S.; Samiey, B.; Tehrani, A.D.; Soleimani, K. Graphene Oxide–Cellulose Nanowhisker Hydrogel Nanocomposite as a Novel Adsorbent for Methylene Blue. J. Chem. Eng. Data 2019, 64, 5558–5570.
- Bhattacharyya, R.; Ray, S.K. Enhanced adsorption of synthetic dyes from aqueous solution by a semi-interpenetrating network hydrogel based on starch. J. Ind. Eng. Chem. 2014, 20, 3714–3725.
- Fang, Y.; Liu, Q.; Zhu, S. Selective biosorption mechanism of methylene blue by a novel and reusable sugar beet pulp cellulose/sodium alginate/iron hydroxide composite hydrogel. Int. J. Biol. Macromol 2021, 188, 993–1002.
- Li, Y.; Hou, X.; Pan, Y.; Wang, L.; Xiao, H. Redox-responsive carboxymethyl cellulose hydrogel for adsorption and controlled release of dye. Eur. Polym. J. 2020, 123, 109447.
- Dai, H.; Huang, Y.; Zhang, Y.; Zhang, H.; Huang, H. Green and facile fabrication of pineapple peel cellulose/magnetic diatomite hydrogels in ionic liquid for methylene blue adsorption. Cellulose 2019, 26, 3825–3844.
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11.
- Lu, W.; Dai, Z.; Li, L.; Liu, J.; Wang, S.; Yang, H.; Cao, C.; Liu, L.; Chen, T.; Zhu, B.; et al. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium(VI). J. Mol. Liq. 2020, 303, 112604.
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211.
- Jamali, M.; Akbari, A. Facile fabrication of magnetic chitosan hydrogel beads and modified by interfacial polymerization method and study of adsorption of cationic/anionic dyes from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105175.
- Hossain, S.; Shahruzzaman, M.; Kabir, S.F.; Rahman, M.S.; Sultana, S.; Mallik, A.K.; Haque, P.; Takafuji, M.; Rahman, M.M. Jute cellulose nanocrystal/poly(N,N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) hybrid hydrogels for removing methylene blue dye from aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 254–263.
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264.
- Zhang, Z.-H.; Xu, J.-Y.; Yang, X.-L. MXene/sodium alginate gel beads for adsorption of methylene blue. Mater. Chem. Phys. 2021, 260, 124123.
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139.
- Isik, B.; Ugraskan, V. Adsorption of methylene blue on sodium alginate-flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167.
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681.
- Malekzadeh, M.; Nejaei, A.; Baneshi, M.M.; Kokhdan, E.P.; Bardania, H. The use of starch-modified magnetic Fe0. nanoparticles for naphthalene adsorption from water samples: Adsorption isotherm, kinetic and thermodynamic studies. Appl. Organomet. Chem. 2018, 32, e4434.
- Guan, Q.F.; Yang, H.B.; Han, Z.M.; Ling, Z.C.; Yin, C.H.; Yang, K.P.; Zhao, Y.X.; Yu, S.H. Sustainable Cellulose-Nanofiber-Based Hydrogels. ACS Nano 2021, 15, 7889–7898.
- Sun, Y.; Zhou, T.; Li, W.; Yu, F.; Ma, J. Amino-functionalized alginate/graphene double-network hydrogel beads for emerging contaminant removal from aqueous solution. Chemosphere 2020, 241, 125110.
- Afzal, M.Z.; Yue, R.; Sun, X.F.; Song, C.; Wang, S.G. Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci. 2019, 543, 76–83.
- Nguyen, H.T.; Phuong, V.N.; Van, T.N.; Thi, P.N.; Dinh Thi Lan, P.; Pham, H.T.; Cao, H.T. Low-cost hydrogel derived from agro-waste for veterinary antibiotic removal: Optimization, kinetics, and toxicity evaluation. Environ. Technol. Innov. 2020, 20, 101098.
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568.
- Karimi, S.; Namazi, H. Magnetic alginate/glycodendrimer beads for efficient removal of tetracycline and amoxicillin from aqueous solutions. Int. J. Biol. Macromol. 2022, 205, 128–140.
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpaa, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429.
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863.




