The pollution and scarcity of freshwater resources are global problems that have a significant influence on human life. It is very important to remove harmful substances in the water to realize the recycling of water resources. Hydrogels have recently attracted attention due to their special three-dimensional network structure, large surface area, and pores, which show great potential for the removal of pollutants in water. In their preparation, natural polymers are one of the preferred materials because of their wide availability, low cost, and easy thermal degradation. However, when it is directly used for adsorption, its performance is unsatisfactory, so it usually needs to be modified in the preparation process. 淡水资源的污染和稀缺是对人类生活产生重大影响的全球性问题。去除水中的有害物质对于实现水资源的循环利用非常重要。水凝胶因其特殊的三维网状结构、较大的比表面积和孔隙而备受关注,显示出去除水中污染物的巨大潜力。在制备过程中,天然聚合物因其广泛的可用性、低成本和易热降解而成为首选材料之一。但是,当它直接用于吸附时,其性能并不理想,因此通常需要在制备过程中进行改性。
- hydrogel
- adsorption
- natural polymer
1. Adsorption Mechanism and Kinetics of Polysaccharide-Based Hydrogels多糖基水凝胶的吸附机理与动力学
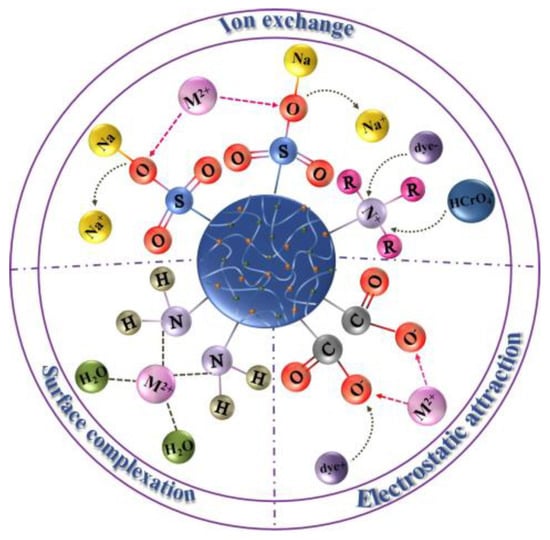
1.1. Adsorption Mechanism
1.1. 吸附机理
1.2. Adsorption Kinetics
1.2. 吸附动力学
1.2.1. Pseudo-First-Order Kinetic伪一阶动力学
1.2.2. Pseudo-Second-Order Kinetic伪二阶动力学
1.3. Adsorption Isotherms
1.3. 吸附等温线
1.3.1. Langmuir Isotherm Equation朗缪尔等温线方程
1.3.2. Freundlich Isotherm Equation弗氏等温线方程
2. Adsorption Applications of Polysaccharide-Based Hydrogels多糖基水凝胶的吸附应用
2.1. Heavy Metal Ion Adsorption
2.1. 重金属离子吸附
| Polysaccharide多糖 Hydrogel水凝胶 Adsorption吸附 |
Adsorbates吸附物 | Adsorption Capacity (mg吸附容量(毫克/g)克) | Adsorption吸附 Isotherm等温线 |
Adsorption吸附 Kinetics动力学 |
Ref.裁判。 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polysaccharide Hydrogels Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. | ||||||
| AM上午/AA | |||||||||||
| C/SA/FeCu铜2+ | 157.51 | MB | 105.93Langmuir朗缪尔 | PSO定位器 | [13][63] | ||||||
| Langmuir | PSO | [88] | Pd钯2+ | 393.28 | Langmuir朗缪尔 | PSO定位器 | |||||
| Cd镉2+ | 289.97 | Langmuir朗缪尔 | PSO定位器 | ||||||||
| Nanocellulose纳米纤维素/Carbon dot碳点 | Cr铬6+ | 599.9 | Freundlich弗罗恩德利希 | PSO定位器 | [14][64] | ||||||
| Straw cellulose秸秆纤维素 | Cd镉2+ | 95.62 | Langmuir朗缪尔 | PSO定位器 | [15][65] | ||||||
| 145 | Langmuir | PSO | [72] | Nanocellulose纳米纤维素/SA南非 | Pb铅2+ | 318.47 | Langmuir朗缪尔 | PSO定位器 | [16][66] | ||
| Millettia speciosa Champ cellulose-CS | CR | 221.43 | Freundlich | PSO | [71] | GO去-PVA-CS | Cd镉2+ | 172.11 | Langmuir朗缪尔 | PSO定位器 | [17][67] |
| Ni镍2+ | 70.37 | Langmuir朗缪尔 | PSO定位器 | ||||||||
| CYCS | |||||||||||
| PAM-Fe3O4-CS | MB | 1603 | Langmuir | PFO | [91] | ||||||
| Montmorillonite-CS | MB | 530 | Langmuir | PSO | [92] | 中国国际铜矿/CNC数控 | Pb铅2+ | 334.92 | Langmuir朗缪尔 | ||
| GO-CS-Fe3O4 | PSO | 定位器 | MB[ | 289 | Langmuir18 | PSO][68] | |||||
| [ | 93 | ] | Chitosan oligosaccharide壳聚糖低聚糖 | Cr铬6+ | 148.1 | ||||||
| EBT | Langmuir | 朗缪尔 | 292PSO定位器 | [19][69] | |||||||
| Langmuir | PSO | [ | α-ketoglutaric acid–PAM酮戊二酸-氨基戊二酸-CS丙烯酸 | Cu铜2+ | 72.39 | Langmuir朗缪尔 | PSO定位器 | [20][50] | |||
| Pd钯2+ | 61.41 | Langmuir朗缪尔 | PSO定位器 | ||||||||
| 94 | ] | ||||||||||
| Jute cellulose nanocrystal | MB | 131.58 | Langmuir | PSO | Zn锌2+ | 51.89 | Langmuir朗缪尔 | PSO定位器 | |||
| CPCS-PAM-PVA | Cr铬6+ | 95.31 | Langmuir朗缪尔 | PFO全氟辛烷磺酸 | [21][70] | ||||||
| Millettia speciosa Champ cellulose-CS | Cu2+ | 23.37 | Freundlich | PFO | [22][71] | ||||||
| All-lignocellulose | Cu2+ | 350 | Langmuir | PSO | [23][72] | ||||||
| Caffeic acid starch | Cr6+ | 96.45 | Langmuir | PSO | [24][73] | ||||||
| Starch-FMBO | As3+ | 161.29 | Langmuir | PSO | [25][74] | ||||||
| Starch nanoparticle | Pb2+ | 40.52 | Langmuir | PSO | [26][27] | ||||||
| Cu2+ | 32.88 | Langmuir | PSO | ||||||||
| dibenzo-18-crown-6 starch | Cd2+ | 368.5 | Freundlich | PSO | [27][75] | ||||||
| Ni2+ | 182.5 | Freundlich | PSO | ||||||||
| Zn2+ | 377.5 | Freundlich | PSO | ||||||||
| Cu2+ | 385 | Freundlich | PSO | ||||||||
| Succinic anhydride-SNCs | Cu2+ | 84.07 | Freundlich | PSO | [28][31] | ||||||
| PVA-SA | ] | [ | 80 | ] | |||||||
| Reptilite-Starch | Pb2+ | 180.8 | Langmuir | PSO | [34][81] | ||||||
| NCDs-CNF/CS | Cu2+ | 148.3 | Langmuir | PSO | [ | ||||||
| Carboxymethylcellulose | MB | 756 | Freundlich | PSO | [12] | ||||||
| Pineapple peel cellulose/diatomite |
MB | 101.94 | Langmuir | PSO | [89] | ||||||
| PCMC-PVA | MB | 172.14 | |||||||||
| Succinic anhydride-SNCs | MB | 84.00 | Freundlich | PSO | [31] | ||||||
| Reptilite-Starch | MB | 277.0 | Langmuir | PSO | [81] | ||||||
| PAM-cassava starch | MB | 2000 | Langmuir | PSO | [95] | ||||||
| MXene-SA | MB | 92.17 | Langmuir | PSO | [96] | ||||||
| AA-GO-SA | MG | 628.93 | Langmuir | PSO | Pb2+ | 784.97 | Langmuir | PSO | [29][76] | ||
| ZIF-67-SA | Cu2+ | 153.63 | Langmuir | PSO | [30][77] | ||||||
| AM-GO-SA | Cu2+ | 68.76 | Langmuir | PSO | [31][78] | ||||||
| [ | 97 | Pb2+ | 240.69 | Langmuir | PSO | ||||||
| Zeolite-PVA-SA | Pb2+ | 99.5 | Langmuir | PFO | [32][79] | ||||||
| Cd2+ | 99.2 | Langmuir | PFO | ||||||||
| Sr2+ | 98.8 | Langmuir | PFO | ||||||||
| Cu2+ | 97.2 | Langmuir | PFO | ||||||||
| Zn2+ | 95.6 | Langmuir | PFO | ||||||||
| Ni2+ | 93.1 | Langmuir | PFO | ||||||||
| Mn2+ | 92.4 | Langmuir | PFO | ||||||||
| ] | Starch ether-SA | Cu2+ | 25.81 | Langmuir | PSO | [3335] | [82] | ||||
| Cr6+ | 294.46 | Langmuir | PSO | ||||||||
| CTS/CA/BT | Pb2+ | 434.89 | Freundlich | PSO | [36][83] | ||||||
| Cu2+ | 115.30 | Freundlich | PSO | ||||||||
| Cd2+ | 102.38 | Freundlich | PSO | ||||||||
| GO-SA | As5+ | 277.39 | Langmuir | PSO | [37][55] | ||||||
| PAN-PPY-SA-GO | Cu2+ | 133.7 | Redlich–Peterson | PFO | [38][84] | ||||||
| Cr6+ | 87.2 | Redlich–Peterson | PFO |
2.2. Dye Adsorption
| Langmuir | |||||
| PSO | |||||
| [ | 90 | ] | |||
| All-lignocellulose | MB | ||||
| Flax seed ash-SA | |||||
| MB | |||||
| 333.3 | |||||
| Langmuir | |||||
| PSO | [ | 98 | ] | ||
| PEI-SA | MB | 400 | Langmuir | PSO | [47] |
| AM-HEMA-Starch | MG | 164 | Langmuir | PFO | [71] |
| MV | 156 | Freundlich | PFO |
| Polysaccharide Hydrogels Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. |
|---|---|---|---|---|---|
| C/SA/Fe | MB | 105.93 | Langmuir | PSO | [43] |
| Carboxymethylcellulose | MB | 756 | Freundlich | PSO | [44] |
| Pineapple peel cellulose/diatomite |
MB | 101.94 | Langmuir | PSO | [45] |
2.3. Drug Antibiotics Adsorption
| Polysaccharide多糖 Hydrogels水凝胶 Adsorption吸附 |
Adsorbates吸附物 | Adsorption Capacity (mg吸附容量(毫克/g)克) | Adsorption吸附 Isotherm等温线 |
Adsorption吸附 Kinetics动力学 |
Ref.裁判。 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe铁3O4-Starch淀粉 | Naphthalene萘 | 24.752 | Langmuir朗缪尔 | PSO定位器 | [56][99] | ||||||
| CS-Chitosan film壳聚糖薄膜 | Cefotaxime Sodium头孢噻肟钠 | 1003.64 | Freundlich弗罗恩德利希 | PSO定位器 | [57][100] | ||||||
| GO-SA戈萨 | Tetracycline四环素 | 477.9 | Freundlich弗罗恩德利希 | PSO定位器 | [37][55] | ||||||
| PCMC-PVA | MB | 172.14 | Langmuir | PSO | [46] | ||||||
| Amino氨基/GO-SA | Ciprofloxacin环丙沙星 | 301.36 | Langmuir朗缪尔 | PSO定位器 | [58][101] | All-lignocellulose | MB | 145 | Langmuir | PSO | [23] |
| Millettia speciosa Champ cellulose-CS | CR | 221.43 | Freundlich | PSO | [22] | ||||||
| PAM-Fe3O4-CS | MB | 1603 | Langmuir | PFO | [47] | ||||||
| Montmorillonite-CS | MB | 530 | Langmuir | ||||||||
| Humicacid腐植酸-CS-Biochar生物炭 | Ciprofloxacin环丙沙星 | 154.89 | Langmuir朗缪尔 | PSO定位器 | [59][102] | ||||||
| Biochar生物炭-CS | Ciprofloxacin环丙沙星 | 106.038 | Langmuir朗缪尔 | PSO定位器 | [60][103] | ||||||
| Enrofloxacin恩诺沙星 | 100.433 | Langmuir朗缪尔 | PSO定位器 | PSO | [48] | ||||||
| GO-SA戈萨 | Fluoxacin氟沙星 | 4.11 | Langmuir朗缪尔 | PSO定位器 | [61][104] | GO-CS-Fe3O4 | MB | 289 | Langmuir | PSO | [49] |
| EBT | 292 | Langmuir | PSO | [50] | |||||||
| Jute cellulose nanocrystal | MB | 131.58 | Langmuir | PSO | |||||||
| Succinic anhydride-SNCs | MB | 84.00 | Freundlich | PSO | [28] | ||||||
| Reptilite-Starch | MB | 277.0 | Langmuir | PSO | [34] | ||||||
| PAM-cassava starch | MB | 2000 | Langmuir | PSO | [51] | ||||||
| MXene-SA | MB | 92.17 | Langmuir | PSO | [52] | ||||||
| AA-GO-SA | MG | 628.93 | Langmuir | PSO | [53] | ||||||
| Flax seed ash-SA | MB | 333.3 | Langmuir | PSO | [54] | ||||||
| Moxifloxacin莫西沙星 | 3.43 | Langmuir朗缪尔 | PSO定位器 | ||||||||
| Fe铁3O4-SA | Tetracycline四环素 | 454.54 | Langmuir朗缪尔 | PSO定位器 | [62][105] | ||||||
| Amoxicillin阿莫西林 | 400 | Langmuir朗缪尔 | PSO定位器 | ||||||||
| Trimethylammonium chloride三甲基氯化铵-CS | Tetracycline四环素 | 22.42 | Langmuir朗缪尔 | PFO全氟辛烷磺酸 | [63][106] | ||||||
| PVA聚氯乙烯-SA-Cu铜2+ | Tetracycline四环素 | 231.431 | Langmuir朗缪尔 | PSO定位器 | [64][107] | PEI-SA | MB | 400 | Langmuir | PSO | [55] |
| AM-HEMA-Starch | MG | 164 | Langmuir | PFO | [22] | ||||||
| MV | 156 | Freundlich | PFO |
