Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
淡水资源的污染和稀缺是对人类生活产生重大影响的全球性问题。去除水中的有害物质对于实现水资源的循环利用非常重要。水凝胶因其特殊的三维网状结构、较大的比表面积和孔隙而备受关注,显示出去除水中污染物的巨大潜力。在制备过程中,天然聚合物因其广泛的可用性、低成本和易热降解而成为首选材料之一。但是,当它直接用于吸附时,其性能并不理想,因此通常需要在制备过程中进行改性。
- hydrogel
- adsorption
- natural polymer
1. 多糖基水凝胶的吸附机理与动力学
水凝胶的吸附可分为化学吸附和物理吸附。化学吸附是一个不可逆的过程,主要是因为它的吸附剂和吸附剂在相互作用中是化学键合的;纽带的破坏是永久性的,一旦被破坏,将无法再次结合。相比之下,物理吸附是一个不可逆的过程,通常由物理力控制,如氢键、离子键、π-π堆积、疏水相互作用等,被破坏后可以恢复。在制备水凝胶时,物理作用通常与化学作用相结合,以加强其吸附性能和机械性能,例如在自修复水凝胶中,通常通过物理作用分散能量消耗,以确保内部化学键和机械性能的完整性得到改善;这种水凝胶广泛用于生物工程[39]。在吸附领域的水凝胶制备中,研究人员还选择将这两种作用模式结合起来。具体的吸附机理及其分类如下。
1.1. 吸附机理
在吸附过程中,不同的官能团具有不同的吸附机理和不同的作用方式,官能团决定了分子间力的类型和强度以及分子的化学反应性。多糖基水凝胶材料吸附过程中涉及的主要官能团可分为三大类:含氧官能团、含氮官能团和含硫官能团。N,O和S类杂原子可以贡献一个或多个电子并与金属离子形成配位键,同时还进行离子交换或静电吸引以实现对各种污染物的吸附。在报道的水凝胶的各种金属吸附机制中,静电相互作用、离子交换和表面络合(包括配位和螯合)这三种方法被发现与表面官能团密切相关[6]。如图1所示,描述了通过这三种作用模式进行吸附时的作用过程,其中两种静电相互作用和离子交换的吸附机理大多是可逆的,吸附剂可以重复使用,属于物理吸附。
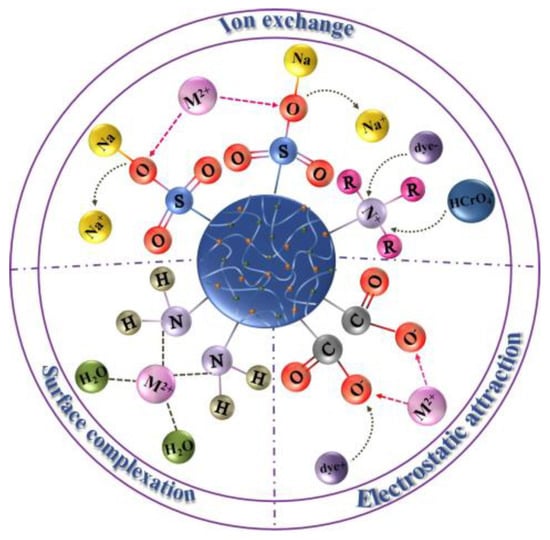
图1.三种模式下的吸附过程示意图。
1.2. 吸附动力学
1.2.1. 伪一阶动力学
伪一级动力学模型基于模态扩散理论,该理论假设吸附剂从溶液到达吸附剂表面受扩散步骤控制,并且吸附剂表面只有一个结合位点[40]。等式的形式如下:
其中 qt是时间T的吸附容量(mg/g),qe是吸附平衡时刻的吸附容量(mg/g),K1是一阶动力学的速率常数。
虽然一阶动力学模型已广泛用于各种吸附过程,但它具有局限性。它通常只适用于吸附初始阶段的动力学描述,不能准确描述整个吸附过程[41]。
1.2.2. 伪二阶动力学
伪二阶动力学模型基于吸附限速步骤,包含吸附机理,如化学吸附,涉及吸附物和吸附剂之间的电子共享或电子转移[42]。与伪二阶动力学模型的一致性表明,吸附动力学主要由化学相互作用控制,而不是由材料传递步骤控制。等式的形式如下:
其中 qt是时间T的吸附容量(mg/g),qe是吸附平衡时刻的吸附容量(mg/g),K2(g/mg·h) 是伪二阶速率常数。
1.3. 吸附等温线
1.3.1. 朗缪尔等温线方程
Langmuir吸附等温线模型是应用最广泛的分子吸附模型,它可以通过考虑吸附剂表面和温度的影响来预测吸附剂的最大吸附容量[43]。该理论是单分子层吸附理论,它要求具有相同吸附容量的均匀固体表面,并且吸附分子之间没有相互作用,但模型的假设与实际情况相去甚远,获得的信息有时非常不准确[44]。等式的形式如下:
其中 Ce是溶液的平衡浓度,mg/L,qm是最大吸附容量(饱和度)、mg/g 和 KL是与键合位点的亲和力和吸附能L/g相关的朗缪尔常数。
C e q e=Ce q m+1qmK L Ceqe=Ceqm+1qmKL
1.3.2. 弗氏等温线方程
弗氏等温吸附方程是一个没有假设的经验方程。等式的形式如下:
其中 qe是吸附达到平衡时的吸附量mg/g,Ce是吸附平衡时溶液中吸附物的浓度,mg/L,KF是弗氏模型下与吸附容量和吸附强度相关的常数,1/n为弗氏常数。K 值大F是吸附剂吸附性能更好的标志。弗氏吸附等温线可通过绘制lnq得到e针对 lnCe在不同温度下[45]。
lnqe=lnK F+1nlnC elnqe=lnKF+1nlnCe
2. 多糖基水凝胶的吸附应用
2.1. 重金属离子吸附
重金属离子剧毒、不可降解,在环境中具有生物蓄积性,在水和生物系统中通过食物链循环,严重影响食物链顶端的生物[59,60]。重金属是密度大于4.5克/厘米的金属3,主要包括金、银、铜、铅、锌、镍、钴、镉、汞、镉等40多种金属[61]。对人体毒性最大的五种是铅、汞、铬、砷和镉。这些重金属在水中不能分解,进入人体时毒性被放大;因此,需要高效和特殊的方法来去除水系统中的重金属污染物[62]。重金属离子的吸附过程与吸附材料本身的官能团直接相关,目前的研究大多围绕二价重金属离子的吸附展开,其中Cu2+, 钯2+和镉2+占主导地位。表1总结了过去两年多糖基吸附材料用于重金属离子吸附的应用。
表 1.不同多糖基复合水凝胶对重金属离子的吸附.
| 多糖 水凝胶 吸附 |
吸附物 | 吸附容量(毫克/克) | 吸附 等温线 |
吸附 动力学 |
裁判。 |
|---|---|---|---|---|---|
| 上午/AA | 铜2+ | 157.51 | 朗缪尔 | PSO定位器 | [63] |
| 钯2+ | 393.28 | 朗缪尔 | PSO定位器 | ||
| 镉2+ | 289.97 | 朗缪尔 | PSO定位器 | ||
| 纳米纤维素/碳点 | 铬6+ | 599.9 | 弗罗恩德利希 | PSO定位器 | [64] |
| 秸秆纤维素 | 镉2+ | 95.62 | 朗缪尔 | PSO定位器 | [65] |
| 纳米纤维素/南非 | 铅2+ | 318.47 | 朗缪尔 | PSO定位器 | [66] |
| 去-PVA-CS | 镉2+ | 172.11 | 朗缪尔 | PSO定位器 | [67] |
| 镍2+ | 70.37 | 朗缪尔 | PSO定位器 | ||
| 中国国际铜矿/数控 | 铅2+ | 334.92 | 朗缪尔 | PSO定位器 | [68] |
| 壳聚糖低聚糖 | 铬6+ | 148.1 | 朗缪尔 | PSO定位器 | [69] |
| α-酮戊二酸-氨基戊二酸-丙烯酸 | 铜2+ | 72.39 | 朗缪尔 | PSO定位器 | [50] |
| 钯2+ | 61.41 | 朗缪尔 | PSO定位器 | ||
| 锌2+ | 51.89 | 朗缪尔 | PSO定位器 | ||
| CPCS-PAM-PVA | 铬6+ | 95.31 | 朗缪尔 | 全氟辛烷磺酸 | [70] |
| Millettia speciosa Champ cellulose-CS | Cu2+ | 23.37 | Freundlich | PFO | [71] |
| All-lignocellulose | Cu2+ | 350 | Langmuir | PSO | [72] |
| Caffeic acid starch | Cr6+ | 96.45 | Langmuir | PSO | [73] |
| Starch-FMBO | As3+ | 161.29 | Langmuir | PSO | [74] |
| Starch nanoparticle | Pb2+ | 40.52 | Langmuir | PSO | [27] |
| Cu2+ | 32.88 | Langmuir | PSO | ||
| dibenzo-18-crown-6 starch | Cd2+ | 368.5 | Freundlich | PSO | [75] |
| Ni2+ | 182.5 | Freundlich | PSO | ||
| Zn2+ | 377.5 | Freundlich | PSO | ||
| Cu2+ | 385 | Freundlich | PSO | ||
| Succinic anhydride-SNCs | Cu2+ | 84.07 | Freundlich | PSO | [31] |
| PVA-SA | Pb2+ | 784.97 | Langmuir | PSO | [76] |
| ZIF-67-SA | Cu2+ | 153.63 | Langmuir | PSO | [77] |
| AM-GO-SA | Cu2+ | 68.76 | Langmuir | PSO | [78] |
| Pb2+ | 240.69 | Langmuir | PSO | ||
| Zeolite-PVA-SA | Pb2+ | 99.5 | Langmuir | PFO | [79] |
| Cd2+ | 99.2 | Langmuir | PFO | ||
| Sr2+ | 98.8 | Langmuir | PFO | ||
| Cu2+ | 97.2 | Langmuir | PFO | ||
| Zn2+ | 95.6 | Langmuir | PFO | ||
| Ni2+ | 93.1 | Langmuir | PFO | ||
| Mn2+ | 92.4 | Langmuir | PFO | ||
| Starch ether-SA | Cu2+ | 25.81 | Langmuir | PSO | [80] |
| Reptilite-Starch | Pb2+ | 180.8 | Langmuir | PSO | [81] |
| NCDs-CNF/CS | Cu2+ | 148.3 | Langmuir | PSO | [82] |
| Cr6+ | 294.46 | Langmuir | PSO | ||
| CTS/CA/BT | Pb2+ | 434.89 | Freundlich | PSO | [83] |
| Cu2+ | 115.30 | Freundlich | PSO | ||
| Cd2+ | 102.38 | Freundlich | PSO | ||
| GO-SA | As5+ | 277.39 | Langmuir | PSO | [55] |
| PAN-PPY-SA-GO | Cu2+ | 133.7 | Redlich–Peterson | PFO | [84] |
| Cr6+ | 87.2 | Redlich–Peterson | PFO |
2.2. Dye Adsorption
There are many methods for removing dyes from wastewater: biological dye removal, acoustic chemical degradation, electrocatalytic degradation, cation exchange membrane technology, etc. However, these processes produce toxic residues that cause secondary pollution and are costly to implement. In contrast, the gel’s adsorption method is simple, efficient, and inexpensive to operate. Hydrogels have a strong dye removal capability, and dyes are more easily diffused in the dissolved hydrogel, which enhances the adsorption capacity via electrostatic interactions with oppositely charged dyes [85].
There is a wide range of adsorbed dyes, among which methylene blue (MB), malachite green (MG), and methyl orange (MO) fuels are more widely adsorbed. MB is a phenothiazine cationic dye, an alkali, that is used to treat methemoglobinemia in histology and microscopy to identify and detect bacteria, to treat fungal infections by staining tissues [21], and to stain cotton and wood. The waste dye discharged into the environment after use can be harmful, causing dizziness, headaches, tremors, and mental confusion, among other symptoms [86]. Malachite green (MG) is a toxic trityl methane chemical, both as a dye and as a bactericidal and parasiticidal chemical, is an alkali, and is prohibited for use in aquaculture. In industries, it is used to color leather, paper, cotton, and silk. However, it is potentially carcinogenic, teratogenic, and mutagenic and is difficult to remove from water [87]. Table 2 summarizes polysaccharide-based adsorbent materials used in the past two years for this application of dye adsorption.
Table 2. Adsorption of dyes by different polysaccharide-based composite hydrogels.
| Polysaccharide Hydrogels Adsorption |
Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm |
Adsorption Kinetics |
Ref. |
|---|---|---|---|---|---|
| C/SA/Fe | MB | 105.93 | Langmuir | PSO | [88] |
| Carboxymethylcellulose | MB | 756 | Freundlich | PSO | [12] |
| Pineapple peel cellulose/diatomite |
MB | 101.94 | Langmuir | PSO | [89] |
| PCMC-PVA | MB | 172.14 | Langmuir | PSO | [90] |
| All-lignocellulose | MB | 145 | Langmuir | PSO | [72] |
| Millettia speciosa Champ cellulose-CS | CR | 221.43 | Freundlich | PSO | [71] |
| PAM-Fe3O4-CS | MB | 1603 | Langmuir | PFO | [91] |
| Montmorillonite-CS | MB | 530 | Langmuir | PSO | [92] |
| GO-CS-Fe3O4 | MB | 289 | Langmuir | PSO | [93] |
| EBT | 292 | Langmuir | PSO | [94] | |
| Jute cellulose nanocrystal | MB | 131.58 | Langmuir | PSO | |
| Succinic anhydride-SNCs | MB | 84.00 | Freundlich | PSO | [31] |
| Reptilite-Starch | MB | 277.0 | Langmuir | PSO | [81] |
| PAM-cassava starch | MB | 2000 | Langmuir | PSO | [95] |
| MXene-SA | MB | 92.17 | Langmuir | PSO | [96] |
| AA-GO-SA | MG | 628.93 | Langmuir | PSO | [97] |
| Flax seed ash-SA | MB | 333.3 | Langmuir | PSO | [98] |
| PEI-SA | MB | 400 | Langmuir | PSO | [47] |
| AM-HEMA-Starch | MG | 164 | Langmuir | PFO | [71] |
| MV | 156 | Freundlich | PFO |
2.3. Drug Antibiotics Adsorption
In the treatment of contaminants in water, attention has been focused mainly on the adsorption of textile dyes and heavy metals. However, the harmful effects of these emerging contaminants, such as pesticides, herbicides, fungicides, pharmaceutical compounds, and personal care products, on the water environment cannot be ignored. Once the toxic substances of pharmaceutical and medical waste enter the soil, they will be adsorbed by the soil, pollute the soil, kill microorganisms and protozoa in the soil, and destroy the microecology in the soil, which in turn will reduce the soil’s ability to degrade pollutants. Furthermore, the acid, alkali, and salts in the substances will change the nature and structure of the soil, leading to the acidification, alkalization, and hardening of the soil, affecting the development and growth of plant roots, and damaging the ecological environment; meanwhile, many harmful drug pollutants can cause serious damage to the liver and nervous systems. Table 3 summarizes the polysaccharide-based adsorption materials used for antibiotic adsorption in the past two years.
Table 3. Adsorption of drug antibiotics by different polysaccharide-based composite hydrogels.
| 多糖 水凝胶 吸附 |
吸附物 | 吸附容量(毫克/克) | 吸附 等温线 |
吸附 动力学 |
裁判。 |
|---|---|---|---|---|---|
| 铁3O4-淀粉 | 萘 | 24.752 | 朗缪尔 | PSO定位器 | [99] |
| CS-壳聚糖薄膜 | 头孢噻肟钠 | 1003.64 | 弗罗恩德利希 | PSO定位器 | [100] |
| 戈萨 | 四环素 | 477.9 | 弗罗恩德利希 | PSO定位器 | [55] |
| 氨基/GO-SA | 环丙沙星 | 301.36 | 朗缪尔 | PSO定位器 | [101] |
| 腐植酸-CS-生物炭 | 环丙沙星 | 154.89 | 朗缪尔 | PSO定位器 | [102] |
| 生物炭-CS | 环丙沙星 | 106.038 | 朗缪尔 | PSO定位器 | [103] |
| 恩诺沙星 | 100.433 | 朗缪尔 | PSO定位器 | ||
| 戈萨 | 氟沙星 | 4.11 | 朗缪尔 | PSO定位器 | [104] |
| 莫西沙星 | 3.43 | 朗缪尔 | PSO定位器 | ||
| 铁3O4-SA | 四环素 | 454.54 | 朗缪尔 | PSO定位器 | [105] |
| 阿莫西林 | 400 | 朗缪尔 | PSO定位器 | ||
| 三甲基氯化铵-CS | 四环素 | 22.42 | 朗缪尔 | 全氟辛烷磺酸 | [106] |
| 聚氯乙烯-SA-铜2+ | 四环素 | 231.431 | 朗缪尔 | PSO定位器 | [107] |
This entry is adapted from the peer-reviewed paper 10.3390/gels9030249
This entry is offline, you can click here to edit this entry!
