
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aubaid Ullah | -- | 5454 | 2023-04-20 04:29:34 | | | |
| 2 | Lindsay Dong | Meta information modification | 5454 | 2023-04-21 03:42:52 | | |
Video Upload Options
Clean methanol can play an important role in achieving net zero emission targets by decarbonizing the energy and chemical sectors. Conventionally, methanol is produced by using fossil fuel as raw material, which releases a significant amount of greenhouse gases (GHGs) into the environment. Clean methanol, which is produced by hydrogen (H2) from renewable sources (green H2) and captured carbon dioxide (CO2), is totally free from the influence of fossil fuel. Due to its vast applications, clean methanol has potential to substitute for fossil fuels while preventing further GHGs emissions. Renewable energy sources such as solar, wind and biomass should be utilized for producing green H2, while CO2 captured from air, and more likely from point emission sources, can be recycled to produce clean methanol. After producing methanol from CO2 and H2, the removal of by-product water by distillation is a big challenge due its high energy consumption. An alternative approach for this methanol-water separation is membrane technology, which is an energy saving option. Water-selective zeolite membranes can separate water post-synthesis, as well as during the synthesis. Production efficiency of methanol can be enhanced by utilizing zeolite membranes inside the methanol synthesis reactor. Furthermore, CO2 conversion as well as methanol selectivity, purity and yield can also be increased significantly by selectively removing by-product water using a zeolite membrane reactor.
1. Feasibility of Methanol as Clean Source of Energy
2. Key Ingredients for Clean Methanol
2.1. Production of Green Hydrogen
About 95% of global hydrogen (H2) is produced from fossil fuel sources, such as natural gas, coal and oil. The production of H2 has been classified into grey, blue and green H2, based on source and production strategy. Grey H2 is produced by steam reforming of fossil fuel and termed as “grey” due to substantial GHGs emissions during the process [47]. The source of blue H2 is also fossil fuel, but the production process must be equipped with efficient CO2 capturing facility (~100%) and have minimal GHGs impact on environment [48]. Green H2 is a totally environmentally-friendly process with zero GHGs emissions [49][50], produced by water electrolysis. The electrolyzer must be powered with renewable energy sources to avoid any GHG emission into the atmosphere during its entire production process [51]. This green H2 is the key approach towards achieving zero emission target of 2050 set by global climate communities. However, despite of the zero emissions from electrolysis operation, an electrolyzer manufacturing process could inevitably lead to GHG emissions, particularly if critical way materials are used, which should be taken into account for net zero emission target [52].
Solid oxide electrolysis (SOEL) is performed in a solid oxide electrolysis cell (SOEC) which works on the reverse principle of a solid oxide fuel cell, used for electricity production from pure H2 and O2 [58]. In SOEC, steam is fed at cathode and it undergoes reduction at high temperatures of 600–1000 °C to produce H2 and oxide ions [59]. Due to the high temperature, oxide ions can travel from cathode to anode via some oxide-conducting electrolyte medium, where it captures an electron from an outer circuit and generates oxygen gas. This process of H2 production has a good efficiency of more than 80% [58], however, it has the drawback of high-temperature operation [60]. To overcome this issue, researchers have made efforts to develop electrolytes for SOEC which can transfer ions at comparatively at low temperatures. Ishihara et al. performed steam electrolysis at 600 °C in SOEC having LaGaO3-based electrolytes and successfully achieved H2 production rate of 70 L/min at 1.8 V of applied potentia [61].
2.1.1. Solar to Hydrogen
-holes (h+) pairs under sunlight irradiation. These e−/h+ pairs migrate towards the active sites of respective electrodes to produce H2 and O2 [65][66][67]. In spite of direct exposure to sunlight in PC technology, the drawbacks include slow kinetics, unfavorable thermodynamics and some safety issues [68]. For instance, the separation of H2/O2 from the PC cell is needed to avoid H2 explosion in contrast with photovoltaic-electrocatalysis (PV-EC) and PEC [69]. Subsequently, research efforts have been focused to overcome these issues for better efficiency of converting solar energy to H2 by improving the semiconductor materials. For example, Wang et al. have developed Pt co-catalyzed BaTaO2N photocatalyst that produced 100 times more efficient H2 production compared to previous studies [70].
2.1.2. Wind to Hydrogen
2.1.3. Biomass to Hydrogen
2.2. Recycled Carbon Dioxide
2.2.1. CO2 from Direct Air Captured
2.2.2. CO2 Captured from Industrial Emissions
Currently, there are three main approaches available to capture CO2 from large-scale fossil fueled industrial sectors: (1) post-combustion capture; (2) pre-combustion capture; and (3) oxy-fuel combustion capture. Among all three technologies, post-combustion carbon capture is the oldest one, due to its ease of operation and installation without any significant changes in existing plant [93]. This technology is based on removal of CO2 from combustion flue gases, mainly consisting of CO2, O2, N2, H2O, SOx and NOx, depending upon the nature of the fuel burned. Around 10–15% of the total volume of flue gases subjected to post-combustion treatment is CO2 [94]. The second technology, pre-combustion carbon capture, refers to the removal of CO2 from fossil fuels before feeding to combustion process [95]. In this technology, fossil fuel is first passed through a gasification process to convert it into syngas (CO, H2 and CO2) which is then subjected to water gas shift reaction to convert CO to CO2 and H2. The final mixture of CO2 and H2 having 15 to 50% CO2, is then fed to a CO2 removal process and clean H2 is used for combustion purpose, while rejecting clean flue gases [96]. Pre-combustion capture technology utilizes a highly concentrated stream of CO2 for carbon capture and hence can perform more efficient removal. The third technology, namely oxy-fuel combustion is one of the leading technologies for carbon capture and utilizes pure oxygen instead of air for fossil fuel burning [97]. An air separation unit separates oxygen from air while leaving behind the major portion of N2. Flue gases produced from this oxy-fuel combustion are more concentrated in CO2, which is then removed from it in a flue gas processing unit [98].
2.2.3. CO2 Produced from Biomass
The gasification of biomass is mainly performed to convert it into clean H2 fuel along with a side product, CO2, which is generated in very abundant amount during this process due to the organic nature of subjected biomass. In this scenario, CO2 is captured from biogas in order to produce value-added H2 fuel [99][100]. Biomass usage is not only limited to H2 production but also used for producing syngas, bio-methane and other light hydrocarbons fuels [101]. In any gasification process, excess CO2 must be removed via either in situ or post process techniques, as it imparts negative value to any produced fuel. Dinca et al. produced syngas by biomass gasification while capturing the CO2 by using liquid absorption method [102]. Dashtestani et al. used novel Ca/Fe based sorbents for efficient removal of CO2 via calcination-carbonation looping from biomass gasification [103]. They also studied the effectiveness of sorbent material over number of regeneration cycles, which is very important stability criteria for a newly developed sorbent.
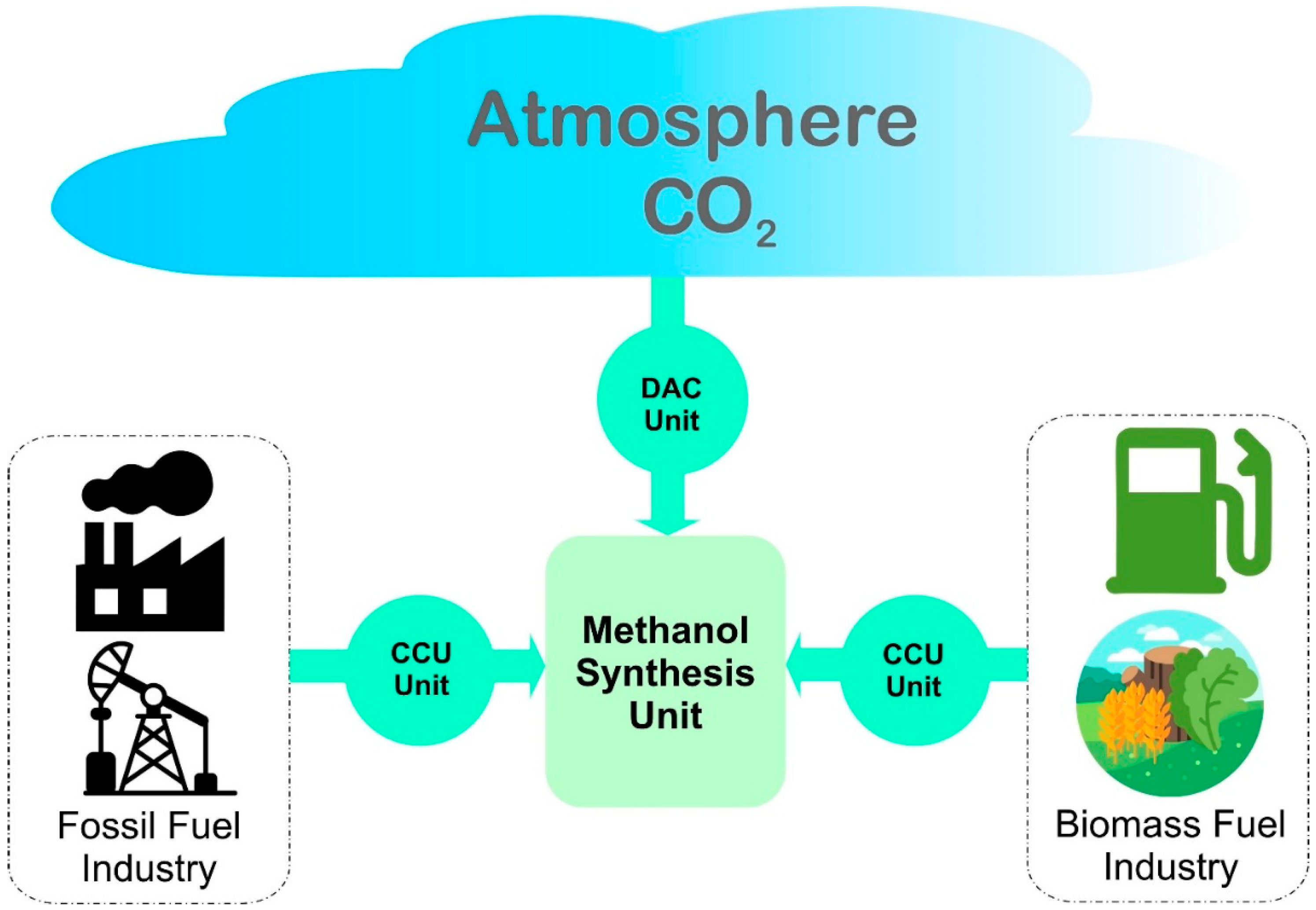
3. Production of Clean Methanol
4. Post-Synthesis Methanol/Water Separation
4.1. Challenges and Improvement in Methanol/Water Separation
4.2. Membrane Based Methanol/Water Separation
4.2.1. Zeolite Membranes for Methanol/Water Separation
4.2.2. Synthesis of Zeolite Membranes
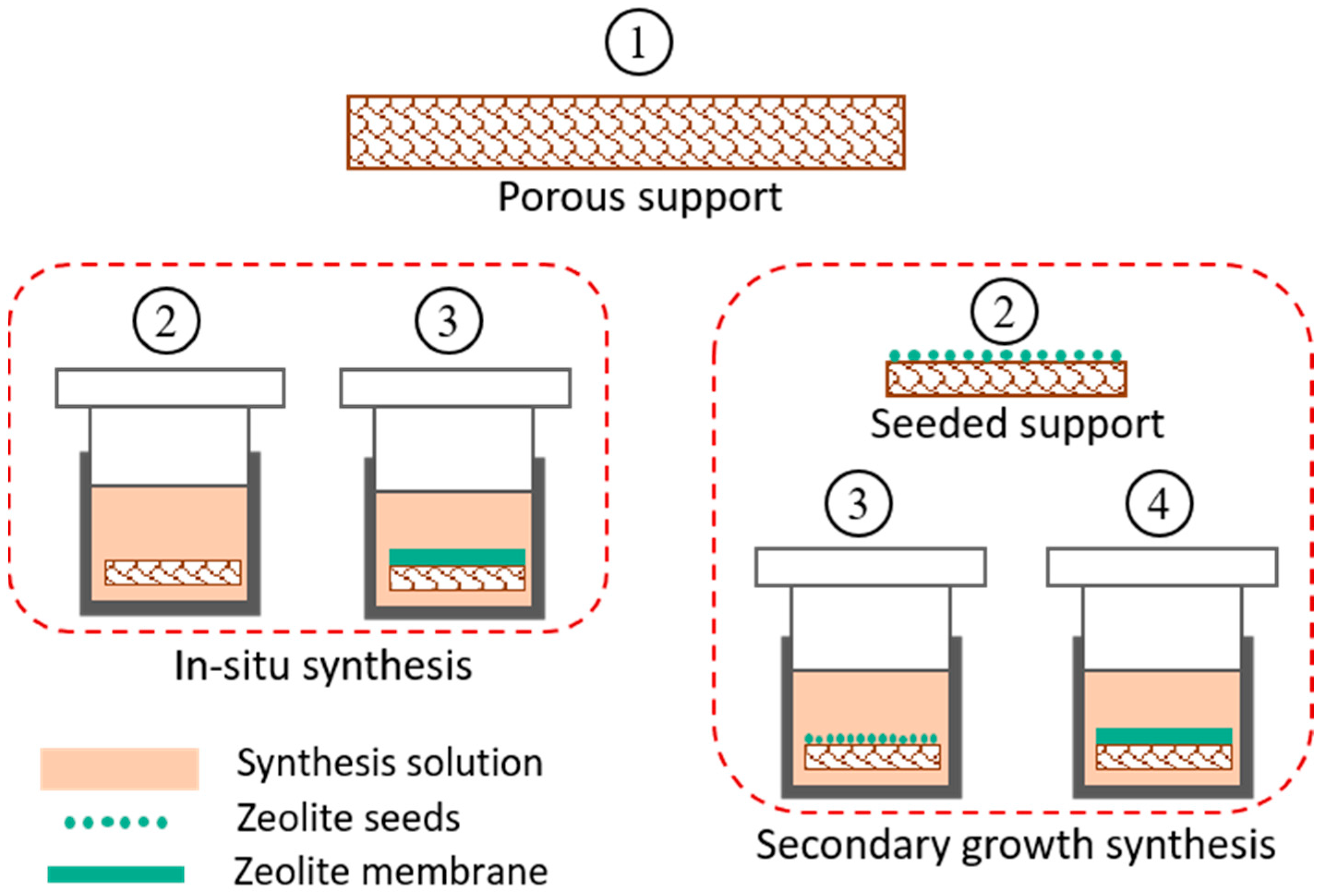
5. Zeolite Membrane Reactors for Methanol Synthesis
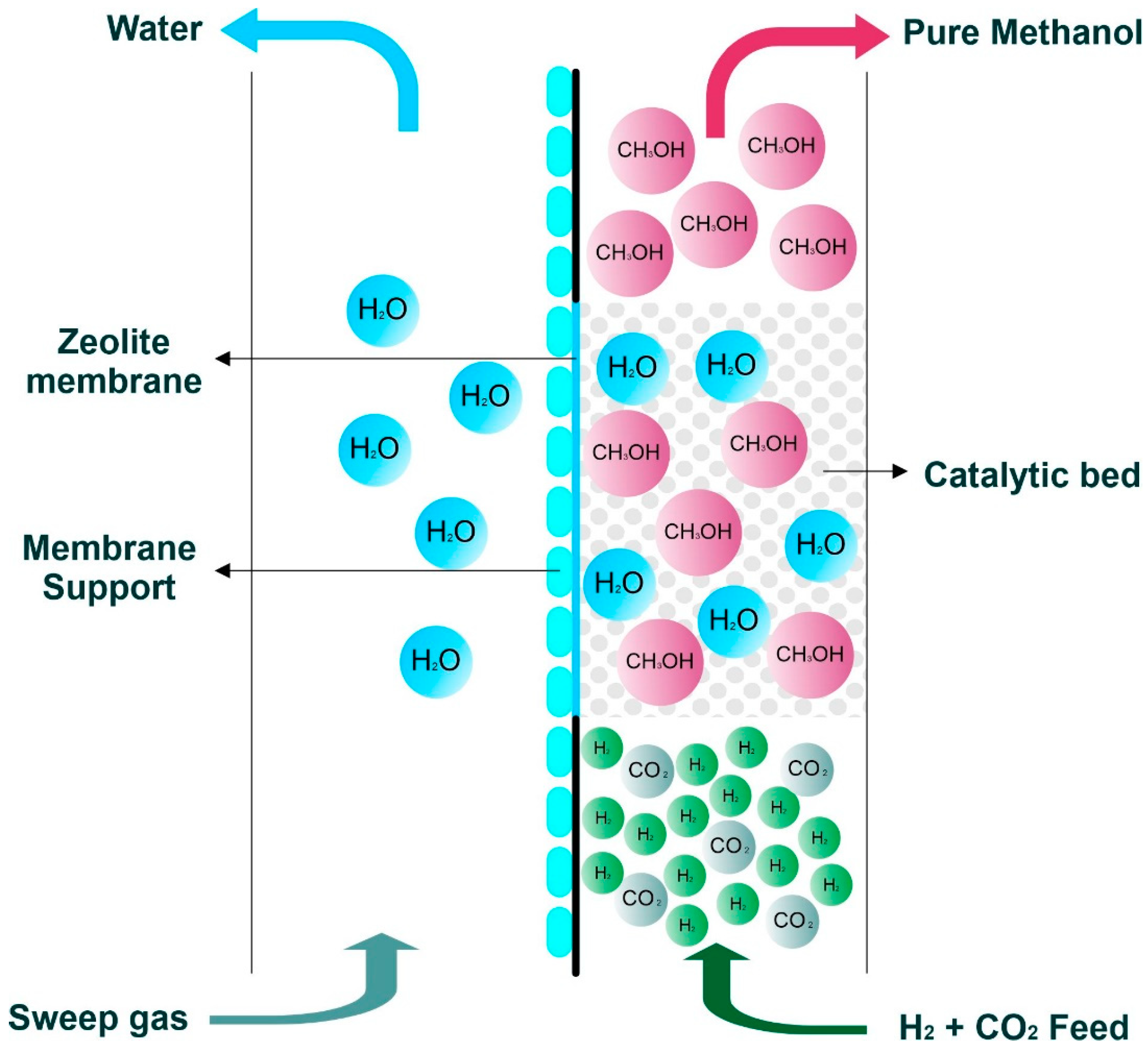
5.1. Effects of In Situ Water Removal on Process Efficiency
5.1.1. Increasing CO2 Conversion and Methanol Yield
5.1.2. In Situ Methanol Purification
References
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Chapter 1—Methanol Production and Applications: An Overview. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28.
- Fernández, L. Production Capacity of Methanol Worldwide from 2018 to 2021—Statista. 2022. Available online: https://www.statista.com/statistics/1065891/global-methanol-production-capacity/#statisticContainer (accessed on 24 November 2022).
- Dalena, F.; Senatore, A.; Basile, M.; Knani, S.; Basile, A.; Iulianelli, A. Advances in Methanol Production and Utilization, with Particular Emphasis toward Hydrogen Generation via Membrane Reactor Technology. Membranes 2018, 8, 98.
- Oner, O.; Dincer, I. Development and assessment of a hybrid biomass and wind energy-based system for cleaner production of methanol with electricity, heat and freshwater. J. Clean. Prod. 2022, 367, 132967.
- Riaz, A.; Zahedi, G.; Klemeš, J.J. A review of cleaner production methods for the manufacture of methanol. J. Clean. Prod. 2013, 57, 19–37.
- Al Hashar, D. Renewable Methanol Production Using Captured Carbon Dioxide and Hydrogen Generated through Water-Splitting. Engineering 2022, 14, 339–359.
- Cifre, P.G.; Badr, O. Renewable hydrogen utilisation for the production of methanol. Energy Convers. Manag. 2007, 48, 519–527.
- González-Garay, A.; Frei, M.S.; Al-Qahtani, A.; Mondelli, C.; Guillén-Gosálbez, G.; Pérez-Ramírez, J. Plant-to-planet analysis of CO2-based methanol processes. Energy Environ. Sci. 2019, 12, 3425–3436.
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical Recycling of Carbon Dioxide to Methanol and Dimethyl Ether: From Greenhouse Gas to Renewable, Environmentally Carbon Neutral Fuels and Synthetic Hydrocarbons. J. Org. Chem. 2009, 74, 487–498.
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.K.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products—Closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048.
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588.
- Dias, V.; Pochet, M.; Contino, F.; Jeanmart, H. Energy and Economic Costs of Chemical Storage. Front. Mech. Eng. 2020, 6, 00021.
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36.
- U.S. Department of Energy. Hydrogen Storage. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 27 October 2022).
- Modi, P.; Aguey-Zinsou, K.-F. Room Temperature Metal Hydrides for Stationary and Heat Storage Applications: A Review. Front. Energy Res. 2021, 9, 616115.
- Tibdewal, S.; Saxena, U.; Gurumoorthy, A. Hydrogen economy vs. Methanol economy. Int. J. Chem. Sci. 2014, 12, 1478–1486.
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous Production of Biodiesel via Transesterification from Vegetable Oils in Supercritical Methanol. Energy Fuels 2006, 20, 812–817.
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511.
- Hosseininejad, S.; Afacan, A.; Hayes, R. Catalytic and kinetic study of methanol dehydration to dimethyl ether. Chem. Eng. Res. Des. 2012, 90, 825–833.
- Methanol Institute. Energy. Available online: https://www.methanol.org/Energy/ (accessed on 3 November 2022).
- Habibic, A. World’s 1st Large-Scale E-Methanol Project to Fuel Maersk’s Boxships. Offshore Energy. 2022. Available online: https://www.offshore-energy.biz/worlds-1st-large-scale-e-methanol-project-to-fuel-maersks-boxships/ (accessed on 21 November 2022).
- Take, T.; Tsurutani, K.; Umeda, M. Hydrogen production by methanol–water solution electrolysis. J. Power Sources 2007, 164, 9–16.
- Menia, S.; Tebibel, H.; Lassouane, F.; Khellaf, A.; Nouicer, I. Hydrogen production by methanol aqueous electrolysis using photovoltaic energy: Algerian potential. Int. J. Hydrogen Energy 2017, 42, 8661–8669.
- Tuomi, S.; Santasalo-Aarnio, A.; Kanninen, P.; Kallio, T. Hydrogen production by methanol–water solution electrolysis with an alkaline membrane cell. J. Power Sources. 2013, 229, 32–35.
- Vázquez, F.V.; Simell, P.; Pennanen, J.; Lehtonen, J. Reactor design and catalysts testing for hydrogen production by methanol steam reforming for fuel cells applications. Int. J. Hydrogen Energy 2016, 41, 924–935.
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021.
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967.
- Zoppi, G.; Pipitone, G.; Gruber, H.; Weber, G.; Reichhold, A.; Pirone, R.; Bensaid, S. Aqueous phase reforming of pilot-scale Fischer-Tropsch water effluent for sustainable hydrogen production. Catal. Today. 2021, 367, 239–247.
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope. Ind. Eng. Chem. Res. 2021, 60, 89–113.
- Stekrova, M.; Rinta-Paavola, A.; Karinen, R. Hydrogen production via aqueous-phase reforming of methanol over nickel modified Ce, Zr and La oxide supports. Catal. Today. 2018, 304, 143–152.
- Pethaiah, S.S.; Sadasivuni, K.K.; Jayakumar, A.; Ponnamma, D.; Tiwary, C.S.; Sasikumar, G. Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies 2020, 13, 5879.
- Ju, H.; Giddey, S.; S.Badwal, P.S.; Mulder, R.J.; Gengenbach, T.R. Methanol-water co-electrolysis for sustainable hydrogen production with PtRu/C-SnO2 electro-catalyst. Ionics 2018, 24, 2367–2378.
- Bahrami, H.; Faghri, A. Review and advances of direct methanol fuel cells: Part II: Modeling and numerical simulation. J. Power Sources 2013, 230, 303–320.
- Radenahmad, N.; Afif, A.; Petra, P.I.; S.Rahman, M.H.; S.-Eriksson, G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358.
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048.
- Lange, J.-P. Methanol synthesis: A short review of technology improvements. Catal. Today 2001, 64, 3–8.
- Van Bennekom, J.; Venderbosch, R.; Winkelman, J.; Wilbers, E.; Assink, D.; Lemmens, K.; Heeres, H. Methanol synthesis beyond chemical equilibrium. Chem. Eng. Sci. 2013, 87, 204–208.
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S. Demonstrating Large Scale Industrial CCS through CCU—A Case Study for Methanol Production. Energy Procedia 2017, 114, 122–138.
- Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34, 13438–13455.
- Sari, D. Methane capture installation for greenhouse gasses emission reduction in palm oil mill. J. Adv. Res. Dyn. Control. Syst. 2019, 11, 459–464.
- Lam, M.K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win–win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141.
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today. 2009, 148, 191–205.
- Do, N.T.Q.; Haag, S.; Castillo-Weltter, F.; Gunther, A. Flexible and Sustainable Methanol Production Including Option with Green Hydrogen. In Proceedings of the 14th International Symposium on Process System Engineering, Kyoto, Japan, 19–23 June 2022; Yamashita, Y., Kano, M., Eds.; pp. 775–780.
- Lonis, F.; Tola, V.; Cau, G. Assessment of integrated energy systems for the production and use of renewable methanol by water electrolysis and CO2 hydrogenation. Fuel 2021, 285, 119160.
- Liquid Wind. Flagships. Available online: https://www.liquidwind.se/flagships (accessed on 19 December 2022).
- Pekic, S. Ørsted Joins Liquid Wind on FlagshipONE E-Methanol Project. 2022. Available online: https://www.offshore-energy.biz/orsted-joins-liquid-wind-on-flagshipone-e-methanol-project/ (accessed on 25 December 2022).
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635.
- Newborough, M.; Cooley, G. Developments in the global hydrogen market: Electrolyser deployment rationale and renewable hydrogen strategies and policies. Fuel Cells Bull. 2020, 2020, 16–22.
- IRENA. Policies for Green Hydrogen. 2020. Available online: https://www.irena.org/Energy-Transition/Policy/Policies-for-green-hydrogen (accessed on 31 October 2022).
- Gerloff, N. Comparative Life-Cycle-Assessment analysis of three major water electrolysis technologies while applying various energy scenarios for a greener hydrogen production. J. Energy Storage 2021, 43, 102759.
- Beswick, R.R.; Oliveira, A.M.; Yan, Y. Does the Green Hydrogen Economy Have a Water Problem? ACS Energy Lett. 2021, 6, 3167–3169.
- Palmer, G.; Roberts, A.; Hoadley, A.; Dargaville, R.; Honnery, D. Life-cycle greenhouse gas emissions and net energy assessment of large-scale hydrogen production via electrolysis and solar PV. Energy Environ. Sci. 2021, 14, 5113–5131.
- Maric, R.; Yu, H. Proton Exchange Membrane Water Electrolysis as a Promising Technology for Hydrogen Production and Energy Storage. In Nanostructures in Energy Generation, Transmission and Storage; Fedorenko, Y., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. 6.
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454.
- Tajuddin, A.A.H.; Wakisaka, M.; Ohto, T.; Yu, Y.; Fukushima, H.; Tanimoto, H.; Li, X.; Misu, Y.; Jeong, S.; Fujita, J.; et al. Corrosion-resistant and high-entropic non-noble-metal electrodes for oxygen evolution in acidic media. Adv. Mater. 2022, 35, 2207466.
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248.
- Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy Environ. Sci. 2019, 12, 2620–2645.
- Wu, J.; Myung, J.; Ding, D.; Zhu, T. Editorial: High Temperature Solid Oxide Cells. Front. Chem. 2021, 9, 719826.
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrogen Energy 2008, 33, 5375–5382.
- Wang, S.; Lu, A.; C.-Zhong, J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4.
- Ishihara, T.; Kanno, T. Steam electrolysis using LaGaO3 based perovskite electrolyte for recovery of unused heat energy. ISIJ Int. 2010, 50, 1291–1295.
- Wang, Z.; Gu, Y.; Wang, L. Revisiting solar hydrogen production through photovoltaic-electrocatalytic and photoelectrochemical water splitting. Front. Energy 2021, 15, 596–599.
- U.S. Department of Energy. Hydrogen Production: Photoelectrochemical Water Splitting. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-photoelectrochemical-water-splitting (accessed on 1 November 2022).
- Grimes, C.A.; Varghese, O.K.; Ranjan, S. Photovoltaic—Electrolysis Cells. In Light, Water, Hydrogen: The Solar Generation of Hydrogen by Water Photoelectrolysis; Grimes, C.A., Varghese, O.K., Ranjan, S., Eds.; Springer: Boston, MA, USA, 2008; pp. 485–516.
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022.
- Takata, T.; Domen, K. Particulate Photocatalysts for Water Splitting: Recent Advances and Future Prospects. ACS Energy Lett. 2019, 4, 542–549.
- Li, Y.; S.Tsang, C.E. Recent progress and strategies for enhancing photocatalytic water splitting. Mater. Today Sustain. 2020, 9, 100032.
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574.
- Li, R.; Li, C. Chapter One—Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–57.
- Wang, Z.; Luo, Y.; Hisatomi, T.; J.Vequizo, J.M.; Suzuki, S.; Chen, S.; Nakabayashi, M.; Lin, L.; Pan, Z.; Kariya, N.; et al. Sequential cocatalyst decoration on BaTaO2N towards highly-active Z-scheme water splitting. Nat. Commun. 2021, 12, 1005.
- How Wind Energy Can Help Clean Hydrogen Contribute to a Zero-Carbon Future. 2022. Available online: https://www.energy.gov/eere/articles/how-wind-energy-can-help-clean-hydrogen-contribute-zero-carbon-future (accessed on 17 November 2022).
- Zhang, Z.; Yan, Y.; Zhang, L.; Ju, S. Hollow fiber membrane contactor absorption of CO2 from the flue gas: Review and perspective. Glob. Int. J. 2014, 16, 355–374.
- Schrotenboer, A.H.; Veenstra, A.A.; Broek, M.A.U.H.; Ursavas, E. A Green Hydrogen Energy System: Optimal control strategies for integrated hydrogen storage and power generation with wind energy. Renew. Sustain. Energy Rev. 2022, 168, 112744.
- Zhang, G.; Wan, X. A wind-hydrogen energy storage system model for massive wind energy curtailment. Int. J. Hydrogen Energy 2014, 39, 1243–1252.
- Douak, M.; Settou, N. Estimation of Hydrogen Production Using Wind Energy in Algeria. Energy Procedia 2015, 74, 981–990.
- Ziazi, R.; Mohammadi, K.; Goudarzi, N. Techno-Economic Assessment of Utilizing Wind Energy for Hydrogen Production Through Electrolysis. In Proceedings of the ASME 2017 Power Conference, Charlotte, NC, USA, 26–30 June 2017.
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117.
- Dalena, F.; Senatore, A.; Tursi, A.; Basile, A. Bioenergy production from second and third generation feedstocks. In Bioenergy Systems for the Future Prospects for Biofuels and Biohydrogen; Elseiver Publishing: London, UK, 2017.
- Liu, J.; Jin, F.; Fan, M.; Zhu, L.; Tang, C.; Chang, R.; Jia, Q.; Li, Q. Production of high-pure hydrogen by an integrated catalytic process: Comparison of different lignocellulosic biomasses and three major components. Fuel 2018, 226, 322–330.
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920.
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579.
- Lopez, G.; Santamaria, L.; Lemonidou, A.; Zhang, S.; Wu, C.; Sipra, A.T.; Gao, N. Hydrogen generation from biomass by pyrolysis. Nat. Rev. Methods Prim. 2022, 2, 20.
- Simell, P.; Kurkela, E.; Ståhlberg, P.; Hepola, J. Catalytic hot gas cleaning of gasification gas. Catal. Today 1996, 37, 459.
- Entesari, N.; Goeppert, A.; Prakash, G.K.S. Renewable methanol synthesis through single step bi-reforming of biogas. Ind. Eng. Chem. Res. 2020, 59, 10542–10551.
- Lindsey, R.; Dlugokencky, E. Climate Change: Atmospheric Carbon Dioxide. 2022. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 19 November 2022).
- NOAA Research News. Global Atmospheric Carbon Dioxide Levels Continue to Rise. 2022. Available online: https://research.noaa.gov/article/ArtMID/587/ArticleID/2914/No-sign-of-significant-decrease-in-global-CO2-emissions (accessed on 20 November 2022).
- Iribarren, D.; Calvo-Serrano, R.; Martín-Gamboa, M.; Galán-Martín, A.; Guillén-Gosálbez, G. Social life cycle assessment of green methanol and benchmarking against conventional fossil methanol. Sci. Total. Environ. 2022, 824, 153840.
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; H.-Henning, M.; Hebling, C. Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide—Power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261.
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current status and pillars of direct air capture technologies. iScience 2022, 25, 103990.
- Budinis, S. Direct Air Capture, Paris. 2022. Available online: https://www.iea.org/reports/direct-air-capture (accessed on 24 November 2022).
- Zhu, X.; Xie, W.; Wu, J.; Miao, Y.; Xiang, C.; Chen, C.; Ge, B.; Gan, Z.; Yang, F.; Zhang, M.; et al. Recent advances in direct air capture by adsorption. Chem. Soc. Rev. 2022, 51, 6574–6651.
- Bravo, J.; Drapanauskaite, D.; Sarunac, N.; Romero, C.; Jesikiewicz, T.; Baltrusaitis, J. Optimization of energy requirements for CO2 post-combustion capture process through advanced thermal integration. Fuel 2021, 283, 118940.
- Rajabloo, T.; Valee, J.; Marenne, Y.; Coppens, L.; de Ceuninck, W. Carbon capture and utilization for industrial applications. Energy Rep. 2023, 9, 111–116.
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63.
- Smith, K.H.; Ashkanani, H.E.; Morsi, B.I.; Siefert, N.S. Physical solvents and techno-economic analysis for pre-combustion CO2 capture: A review. Int. J. Greenh. Gas Control 2022, 118, 103694.
- Cebrucean, D.; Ionel, I. 5.15—Biomass Co-Firing with Carbon Capture. In Comprehensive Renewable Energy, 2nd ed.; Letcher, E., Ed.; Elsevier: Oxford, UK, 2022; pp. 330–347.
- Koohestanian, E.; Shahraki, F. Review on principles, recent progress, and future challenges for oxy-fuel combustion CO2 capture using compression and purification unit. J. Environ. Chem. Eng. 2021, 9, 105777.
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125.
- Inayat, A.; Ahmad, M.M.; Yusup, S.; Mutalib, M.I.A. Biomass steam gasification with in-situ co2 capture for enriched hydrogen gas production: A reaction kinetics modelling approach. Energies 2010, 3, 1472–1484.
- Detchusananard, T.; Im-orb, K.; Ponpesh, P.; Arpornwichanop, A. Biomass gasification integrated with co2 capture processes for high-purity hydrogen production: Process performance and energy analysis. Energy Convers. Manag. 2018, 171, 1560–1572.
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248.
- Dinca, C.; Slavu, N.; Cormoş, C.C.; Badea, A. CO2 capture from syngas generated by a biomass gasification power plant with chemical absorption process. Energy 2018, 149, 925–936.
- Dashtestani, F.; Nusheh, M.; Siriwongrungson, V.; Hongrapipat, J.; Materic, V.; Pang, S. CO2 capture from biomass gasification producer gas using a novel calcium and iron-based sorbent through carbonation–calcination looping. Ind. Eng. Chem. Res. 2020, 59, 18447–18459.
- Wich, T.; Lueke, W.; Deerberg, G.; Oles, M. Carbon2Chem®-CCU as a Step Toward a Circular Economy. Front. Energy Res. 2020, 7, 00162. Available online: https://www.frontiersin.org/articles/10.3389/fenrg.2019.00162 (accessed on 3 January 2023).
- Li, P.; Gong, S.; Li, C.; Liu, Z. Analysis of routes for electrochemical conversion of CO2 to methanol. Clean Energy 2022, 6, 202–210.
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413.
- Zhang, X.; Zhang, G.; Song, C.; Guo, X. Catalytic Conversion of Carbon Dioxide to Methanol: Current Status and Future Perspective. Front. Energy Res. 2021, 8, 621119. Available online: https://www.frontiersin.org/articles/10.3389/fenrg.2020.621119 (accessed on 3 January 2023).
- Wang, Y.; Gao, W.; Li, K.; Zheng, Y.; Xie, Z.; Na, W.; Chen, J.G.; Wang, H. Strong Evidence of the Role of H2O in Affecting Methanol Selectivity from CO2 Hydrogenation over Cu-ZnO-ZrO2. Chem 2020, 6, 419–430.
- Allam, D.; Bennici, S.; Limousy, L.; Hocine, S. Improved Cu- and Zn-based catalysts for CO2 hydrogenation to methanol. Comptes Rendus Chim. 2019, 22, 227–237.
- Thi, H.T.D.; Mizsey, P.; Toth, A.J. Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes. Membranes 2020, 10, 345.
- Scharzec, B.; Merschhoff, D.; Henrichs, J.; Kappert, E.J.; Skiborowski, M. Evaluation of membrane-assisted hybrid processes for the separation of a tetrahydrofuran-methanol-water mixture. Chem. Eng. Process. Process Intensif. 2021, 167, 108545.
- Liu, S.; Cui, C.; Sun, J. A Novel Synergistic 4-column Methanol Distillation Process. Chem. Eng. Trans. 2017, 61, 937–942.
- Halager, N.S.; Bayer, C.; Kirkpatrick, R.; Gernaey, K.V.; Huusom, J.K.; Udugama, I.A. Modelling and control of an integrated high purity methanol distillation configuration. Chem. Eng. Process. Process Intensif. 2021, 169, 108640.
- Jana, A.K. Heat integrated distillation operation. Appl. Energy 2010, 87, 1477–1494.
- Kiss, A.A.; Landaeta, S.J.F.; Ferreira, C.A.I. Towards energy efficient distillation technologies—Making the right choice. Energy 2012, 47, 531–542.
- Suphanit, B. Design of internally heat-integrated distillation column (HIDiC): Uniform heat transfer area versus uniform heat distribution. Energy 2010, 35, 1505–1514.
- Kim, Y. Energy Conservation of a Multi-Effect Distillation Column with Internal Heat Integration. J. Chem. Eng. Jpn. 2012, 45, 840–849.
- Zhang, J.; Liang, S.; Feng, X. A novel multi-effect methanol distillation process. Chem. Eng. Process. Process Intensif. 2010, 49, 1031–1037.
- Liang, K.; Li, W.; Luo, H.; Xia, M.; Xu, C. Energy-Efficient Extractive Distillation Process by Combining Preconcentration Column and Entrainer Recovery Column. Ind. Eng. Chem. Res. 2014, 53, 7121–7131.
- Bano, S.; Mahmood, A.; Lee, K.-H. Vapor Permeation Separation of Methanol–Water Mixtures: Effect of Experimental Conditions. Ind. Eng. Chem. Res. 2013, 52, 10450–10459.
- Hu, K.; Nie, J.; Liu, J.; Zheng, J. Separation of methanol from methanol/water mixtures with pervaporation hybrid membranes. J. Appl. Polym. Sci. 2013, 128, 1469–1475.
- Vane, L.M. Review: Membrane Materials for the Removal of Water from Industrial Solvents by Pervaporation and Vapor Permeation. J. Chem. Technol. Biotechnol. 2019, 94, 343–365.
- Vane, L.M. Review of pervaporation and vapor permeation process factors affecting the removal of water from industrial solvents. J. Chem. Technol. Biotechnol. 2020, 95, 495–512.
- Khaleque, A.; Alam, M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir, M.; Karmakar, A.K.; Zhou, J.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019.
- Liu, Q.; Noble, R.D.; Falconer, J.L.; Funke, H.H. Organics/water separation by pervaporation with a zeolite membrane. J. Memb. Sci. 1996, 117, 163–174.
- Aoki, K.; Kusakabe, K.; Morooka, S. Separation of Gases with an A-Type Zeolite Membrane. Ind. Eng. Chem. Res. 2000, 39, 2245–2251.
- Peng, L.; Wu, Z.; Wang, B.; Liu, H.; Zhang, C.; Gu, X. Fabrication of high-stability W-MFI zeolite membranes for ethanol/water mixture separation. J. Memb. Sci. 2022, 659, 120729.
- Sawamura, K.; Furuhata, T.; Sekine, Y.; Kikuchi, E.; Subramanian, B.; Matsukata, M. Zeolite Membrane for Dehydration of Isopropylalcohol–Water Mixture by Vapor Permeation. ACS Appl. Mater. Interfaces 2015, 7, 13728–13730.
- Jiang, J.; Wang, X.; Zhang, Y.; Liu, D.; Gu, X. Fabrication of pure-phase CHA zeolite membranes with ball-milled seeds at low K+ concentration. Microporous Mesoporous Mater. 2015, 215, 98–108.
- Bedard, R.; Liu, C. Recent Advances in Zeolitic Membranes. Annu. Rev. Mater. Res. 2018, 48, 83–110.
- Eterigho-Ikelegbe, O.; Bada, S.; Daramola, M. Preparation and Evaluation of Nanocomposite Sodalite/α-Al2O3 Tubular Membranes for H2/CO2 Separation. Membranes 2020, 10, 312.
- Yan, Y.; Davis, M.E.; Gavalas, G.R. Preparation of Zeolite ZSM-5 Membranes by In-Situ Crystallization on Porous .alpha.-Al2O3. Ind. Eng. Chem. Res. 1995, 34, 1652–1661.
- Geus, E.R.; Exter, M.J.D.; van Bekkum, H. Synthesis and characterization of zeolite (MFI) membranes on porous ceramic supports. J. Chem. Soc. Faraday Trans. 1992, 88, 3101–3109.
- Schneider, H.; Schindel, L.K.; Gomes, L.B.; Tessaro, I.C.; Marcilio, N.R. Template-free ZSM-5 membrane preparation on alumina support by secondary hydrothermal synthesis. Curr. Res. Green Sustain. Chem. 2021, 4, 100049.
- Algieri, C. Secondary Growth Method for Zeolite Membrane Preparation. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–2.
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 229.
- Matsukata, M.; Sekine, Y.; Kikuchi, E.; Sakai, M.; Subramanian, B.; Toyoda, M.; Furuhata, T. Synthesis of FAU-Zeolite Membrane by a Secondary Growth Method: Influence of Seeding on Membrane Growth and Its Performance in the Dehydration of Isopropyl Alcohol–Water Mixture. ACS Omega 2021, 6, 9834–9842.
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxy sodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896.
- Arepalli, D.; Rehman, A.-U.; Kim, M.-Z.; Alam, S.F.; Cho, C.-H. Optimal synthesis of nanosize seeds for secondary growth of high performance hydroxy-sodalite (H-SOD) zeolite membranes for small gas and water separations. Microporous Mesoporous Mater. 2021, 329, 111451.
- Li, H.; Qiu, C.; Ren, S.; Dong, Q.; Zhang, S.; Zhou, F.; Liang, X.; Wang, J.; Li, S.; Yu, M. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367, 667–671.
- Nazir, L.S.M.; Yeong, Y.F.; Chew, T.L. Methods and synthesis parameters affecting the formation of FAU type zeolite membrane and its separation performance: A review. J. Asian Ceram. Soc. 2020, 8, 553–571.
- Li, Z.; Deng, Y.; Dewangan, N.; Hu, J.; Wang, Z.; Tan, X.; Liu, S.; Kawi, S. High Temperature Water Permeable Membrane Reactors for CO2 Utilization. Chem. Eng. J. 2021, 420, 129834.
- Yue, W.; Li, Y.; Wei, W.; Jiang, J.; Caro, J.; Huang, A. Highly Selective CO2 Conversion to Methanol in a Bifunctional Zeolite Catalytic Membrane Reactor. Angew. Chem. Int. Ed. 2021, 60, 18289–18294.
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process Intensif. 2004, 43, 1029–1036.
- Struis, R.; Stucki, S. Verification of the membrane reactor concept for the methanol synthesis. Appl. Catal. A Gen. 2001, 216, 117–129.
- Struis, R.; Stucki, S.; Wiedorn, M. A membrane reactor for methanol synthesis. J. Memb. Sci. 1996, 113, 93–100.
- Chen, G.; Yuan, Q. Methanol synthesis from CO2 using a silicone rubber/ceramic composite membrane reactor. Sep. Purif. Technol. 2004, 34, 227–237.
- Biswal, T.; Shadangi, K.P.; Sarangi, P.K.; Srivastava, R.K. Conversion of carbon dioxide to methanol: A comprehensive review. Chemosphere 2022, 298, 134299.
- Borisut, P.; Nuchitprasittichai, A. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization. Front. Energy Res. 2019, 7, 81.
- Gallucci, F.; Fernandez, E.; Corengia, P.; Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66.
- Diban, N.; Aguayo, A.T.; Bilbao, J.; Urtiaga, A.; Ortiz, I. Membrane Reactors for in Situ Water Removal: A Review of Applications. Ind. Eng. Chem. Res. 2013, 52, 10342–10354.
- Seshimo, M.; Liu, B.; Lee, H.; Yogo, K.; Yamaguchi, Y.; Shigaki, N.; Mogi, Y.; Kita, H.; Nakao, S.-I. Membrane Reactor for Methanol Synthesis Using Si-Rich LTA Zeolite Membrane. Membranes 2021, 11, 505.




